(T) Primary Tumor | Adapted from 7th edition AJCC Staging Forms. | |||
TNM | FIGO | Definitions | ||
TX | Primary tumor cannot be assessed | |||
T0 | No evidence of primary tumor | |||
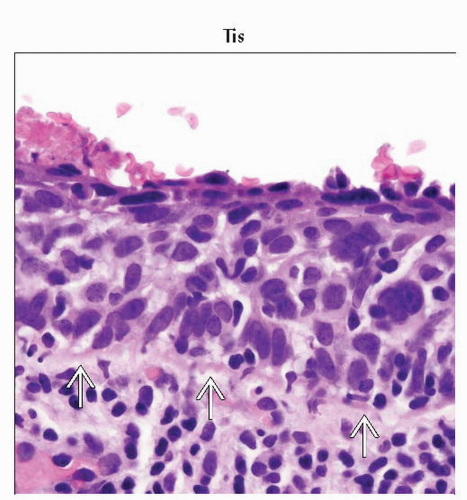
Tis1 | Carcinoma in situ (preinvasive carcinoma) | |||
T1 | I | Cervical carcinoma confined to uterus (extension to corpus should be disregarded) | ||
T1a2 | IA | Invasive carcinoma diagnosed only by microscopy; stromal invasion with a maximum | ||
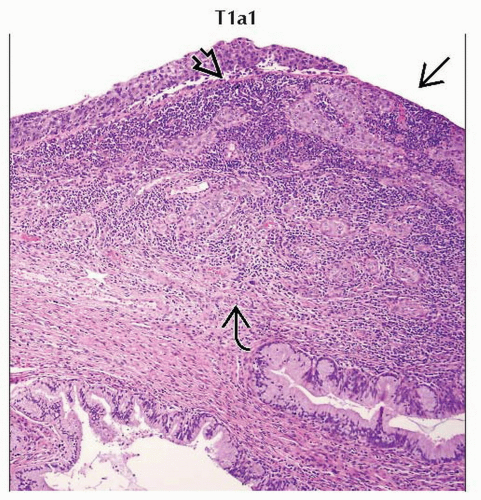
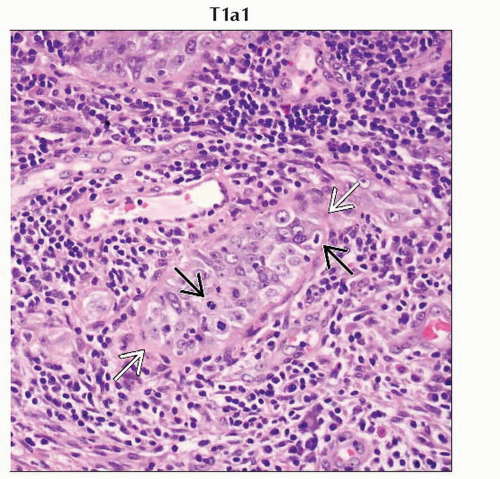
T1a1 | IA1 | Measured stromal invasion ≤ 3.0 mm in depth and ≤ 7.0 mm in horizontal spread | ||
T1a2 | IA2 | Measured stromal invasion > 3.0 mm and ≤ 5.0 mm with a horizontal spread ≤ 7.0 mm | ||
T1b | IB | Clinically visible lesion confined to the cervix or microscopic lesions greater than T1a/IA2 | ||
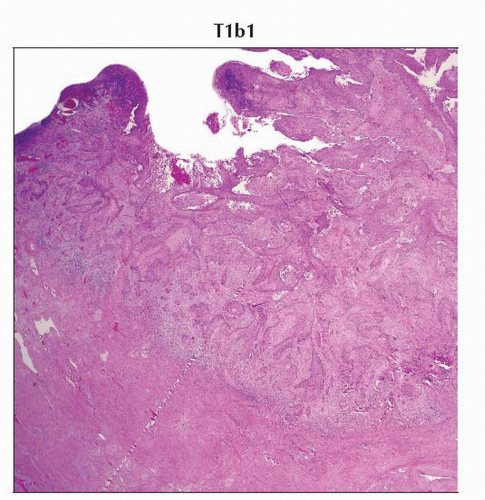
T1b1 | IB1 | Clinically visible lesion ≤ 4.0 cm in greatest dimension | ||
T1b2 | IB2 | Clinically visible lesion > 4.0 cm in greatest dimension | ||
T2 | II | Cervical carcinoma invades beyond uterus but not to pelvic wall or to lower 1/3 of | ||
T2a | IIA | Tumor without parametrial invasion | ||
T2a1 | IIA1 | Clinically visible lesion ≤ 4.0 cm in greatest dimension | ||
T2a2 | IIA2 | Clinically visible lesion > 4.0 cm in greatest dimension | ||
T2b | IIB | Tumor with parametrial invasion | ||
T3 | III | Tumor extends to pelvic wall &/or involves lower 1/3 of vagina, &/or causes | ||
T3a | IIIA | Tumor involves lower 1/3 of vagina, no extension to pelvic wall | ||
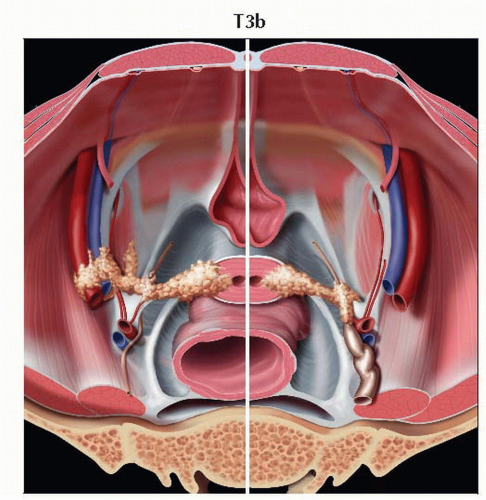
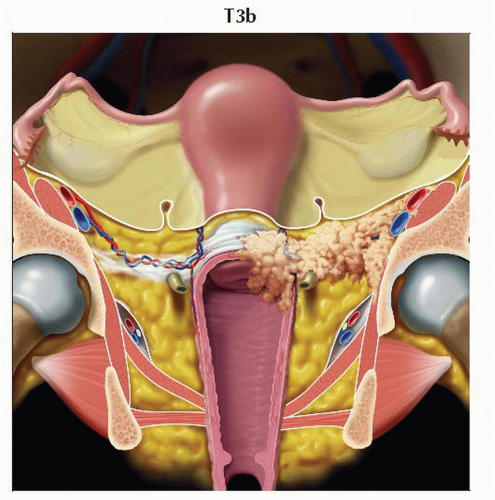
T3b | IIIB | Tumor extends to pelvic wall &/or causes hydronephrosis or nonfunctioning kidney | ||
T4 | IVA | Tumor invades mucosa of bladder or rectum, &/or extends beyond true pelvis (bullous | ||
(N) Regional Lymph Nodes | ||||
NX | Regional lymph nodes cannot be assessed | |||
N0 | No regional lymph node metastasis | |||
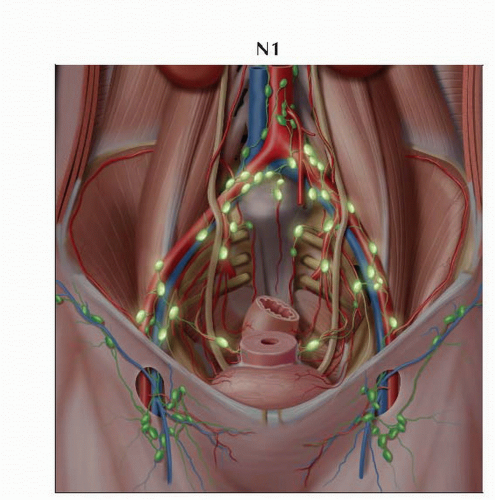
N1 | IIIB | Regional lymph node metastasis | ||
(M) Distant Metastasis | ||||
M0 | No distant metastasis | |||
M1 | IVB | Distant metastasis (including peritoneal spread, involvement of supraclavicular, | ||
1 FIGO no longer includes stage 0 (Tis). | ||||
AJCC Stages/Prognostic Groups | Adapted from 7th edition AJCC Staging Forms. | ||||
Stage | T | N | M | ||
0 | Tis | N0 | M0 | ||
I | T1 | N0 | M0 | ||
IA | T1a | N0 | M0 | ||
IA1 | T1a1 | N0 | M0 | ||
IA2 | T1a2 | N0 | M0 | ||
IB | T1b | N0 | M0 | ||
IB1 | T1b1 | N0 | M0 | ||
IB2 | T1b2 | N0 | M0 | ||
II | T2 | N0 | M0 | ||
IIA | T2a | N0 | M0 | ||
IIA1 | T2a1 | N0 | M0 | ||
IIA2 | T2a2 | N0 | M0 | ||
IIB | T2b | N0 | M0 | ||
III | T3 | N0 | M0 | ||
IIIA | T3a | N0 | M0 | ||
IIIB | T3b | Any N | M0 | ||
T1-3 | N1 | M0 | |||
IVA | T4 | Any N | M0 | ||
IVB | Any T | Any N | M1 | ||
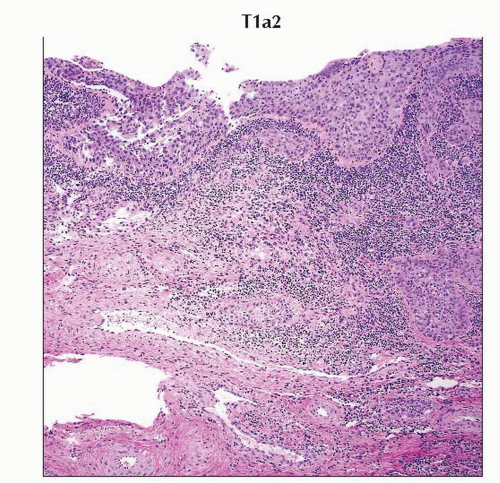 H&E section of the cervix with stromal depth of invasion of 3.5 mm is characteristic of tumor stage T1a2. (Original magnification 40x.) |
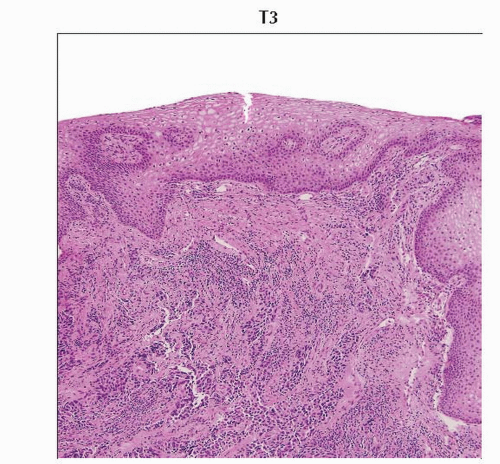 Low-power magnification of H&E stained slide shows cervical squamous cell carcinoma involving the lower 1/3 of the vagina. (Original magnification 40x.) |
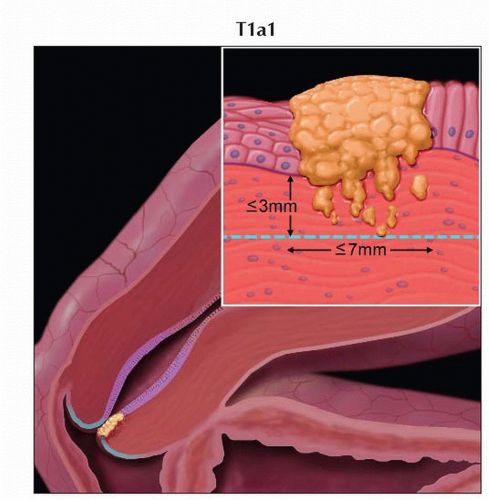 Stage T1a1 cervical carcinoma is defined as microscopic tumor with stromal invasion of ≤ 3 mm in depth and ≤ 7 mm in horizontal spread. |
 Stage T1a2 cervical carcinoma is microscopic tumor with stromal invasion of 4-5 mm in depth and ≤ 7 mm in horizontal spread. |
 Stage T3a tumor invades the lower 1/3 of the vagina. Graphic is a sagittal view of the pelvis showing tumor invading the lower vagina. |
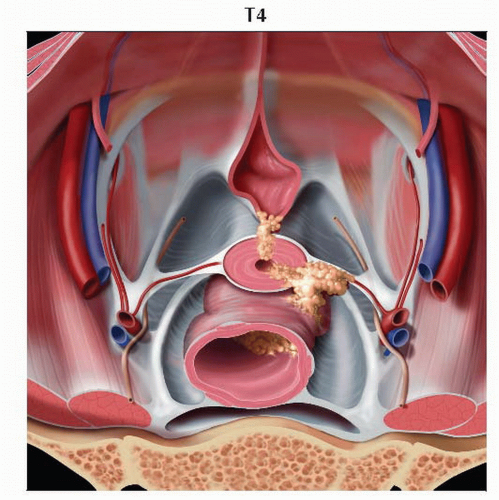 Stage T4 tumor invades the urinary bladder or rectal mucosa. Graphic looks into the pelvic bowl and shows tumors invading the urinary bladder mucosa anteriorly and the rectal mucosa posteriorly. |
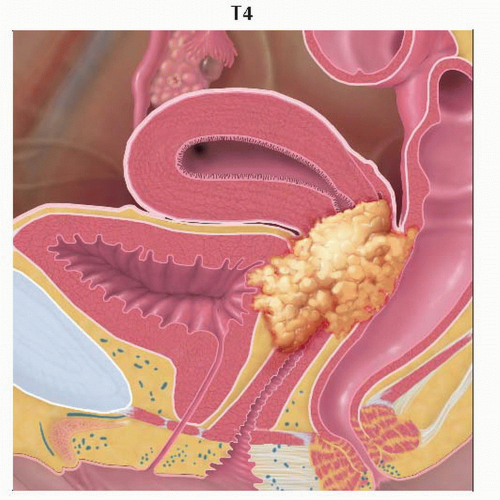 Stage T4 tumor invades the urinary bladder or rectal mucosa. Graphic is a sagittal view of the pelvis showing tumor invading the urinary bladder mucosa anteriorly and the rectal mucosa posteriorly. |
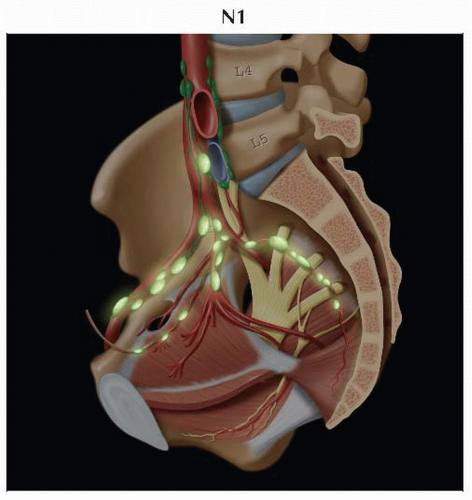 Lateral view of the pelvis shows the presacral and hypogastric routes of lymphatic drainage more clearly. The obturator lymph node, often the sentinel node in cervical carcinoma, is also shown. |
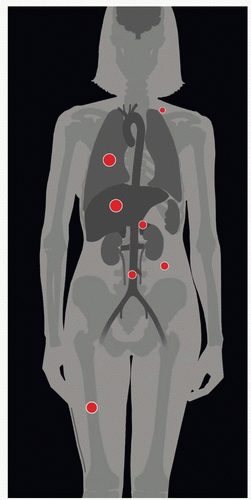
| METASTASES, ORGAN FREQUENCY | |
Liver | 33% | |
Pulmonary | 33-38% | |
Bone | 15-29% | |
Adrenal gland | 15% | |
Paraaortic lymph nodes | 15% | |
Supraclavicular nodes | 7% | |
Abdominal cavity | 5-27% | |
Reported organ frequency of metastatic disease is based on | ||
3rd most common gynecologic malignancy
80% are squamous cell carcinoma
Histopathologic types
Cervical intraepithelial neoplasia, grade III
Squamous cell carcinoma in situ
Squamous cell carcinoma
Invasive
Keratinizing
Nonkeratinizing
Verrucous
Adenocarcinoma in situ
Invasive adenocarcinoma
Endometrioid adenocarcinoma
Clear cell adenocarcinoma
Adenosquamous carcinoma
Adenoid cystic carcinoma
Adenoid basal cell carcinoma
Small cell carcinoma
Neuroendocrine
Undifferentiated carcinoma
Contiguous spread
Most common mode of spread
Caudally to invade
Vagina
Anteriorly to invade
Vesicouterine ligament
Urinary bladder
Laterally to invade
Cardinal ligaments
Paracervical tissues
Fat, vessels, ureters, lymphatics
Pelvic sidewall in advanced disease
Iliac vessels, pelvic musculature
Posteriorly to invade
Uterosacral ligaments
Rectum
Lymphatic spread
Significant prognostic indicator
↑ incidence with advancing stage of disease
Correlates with ↓ disease-free survival
↑ incidence of recurrence at each stage with lymphatic invasion
Lymphatic drainage of cervix
Parametrial → obturator → internal/external iliac
3 pathways of lymphatic drainage of cervix
Lateral route
Parallels external iliac vessels
Tumor drains 1st to medial external iliac chain, then to middle and lateral chains
Deep inguinal lymph nodes drain via lateral route
Hypogastric route
Parallels internal iliac vessels
Lymph nodes along internal iliac branches drain to junctional lymph nodes
Junctional lymph nodes lie between internal and external iliac vessels
Presacral route
Along uterosacral ligament
Uterosacral ligament → lymphatic plexus anterior to sacrum
All 3 routes of lymphatic drainage of cervix drain to common iliac chains
Common iliac chains drain to paraaortic lymph nodes
Depth of invasion of cervix and adjacent structures may affect nodal involvement
Parametrial and pelvic sidewall invasion
Drainage by external iliac lymph nodes
Invasion of lower 1/3 of vagina
Inguinal lymph node metastases
Rectal wall invasion
Drainage by inferior mesenteric lymph nodes
Peritoneal seeding
Peritoneal metastasis varies from 5-27% in autopsy series
Mesenteric or omental metastases are uncommon
“Sister Joseph” nodule
Umbilical metastasis
Direct extension of tumor from anterior peritoneal surface
Hematogenous spread
Liver is most common abdominal organ with metastases
Adrenal gland is 2nd most common metastatic site in abdomen
Pulmonary metastases are relatively common in autopsy series (33-38%)
May be present for significant period of time; however, may remain asymptomatic
1/3 will have mediastinal or hilar adenopathy
Lymphangitic carcinomatosis occurs in < 5%
•Comments
Cervical cancer originates at squamocolumnar junction (SCJ)
SCJ is originally located in ectocervix (intravaginal)
SCJ moves to endocervix with advancing age
Cancer arises in transformation zone between old and new SCJ
Migration of SCJ accounts for age-related change in tumor growth pattern
Young women: Exophytic growth
Older women: Endophytic growth
Adenocarcinoma and small cell cervical cancer
Aggressive histologic subtypes
More often advanced at presentation
Adenoma malignum
Subtype of adenocarcinoma (3%)
Arises from columnar epithelium of endocervical canal
Composed of well-differentiated endocervical glands
History of copious watery discharge
Prognosis is poor
Associated with Peutz-Jeghers syndrome
Clear cell adenocarcinoma
Rare histologic subtype of adenocarcinoma
Associated with in utero diethylstilbestrol (DES) exposure
Case reports suggest possible association with cervical endometriosis
Etiology
Risk factors for cervical cancer
High-risk strains of human papilloma virus (HPV)
Sexual activity at early age
Multiple sexual partners
Sexually transmitted disease
Multiparity
Low socioeconomic status
Cigarette smoking
Immunosuppression
Long-term use of oral contraceptives
In utero DES exposure
Clear cell adenocarcinoma
70% of cervical cancer is caused by HPV 16 and 18
27% of women in USA age 14-59 years are positive for at least 1 strain of HPV
15.2% are positive for 1 of high-risk strains
Women with HIV/AIDS have poor prognosis, often rapidly progressive cancer
Epidemiology & cancer incidence
3rd most common gynecologic malignancy following endometrial and ovarian cancer
Decreased incidence since introduction and widespread use of Papanicolaou smear
Estimated 11,270 women diagnosed in 2009 in the USA
Estimated 4,070 cervical cancer-related deaths in 2009 in the USA
Gross appearance
Poorly circumscribed granular or eroded appearance
Nodular, ulcerated lesion or exophytic mass
Diffuse enlargement and hardening of cervix
Endophytic infiltrative lesion in cervical canal
Barrel-shaped cervix
Diffusely enlarged, bulky, and > 6 cm
Most common with adenocarcinoma
H&E
Large-cell keratinizing squamous cell carcinoma
Sheets & nests of malignant squamous cells invade stroma
Abundant cytoplasm
Large pleomorphic nuclei & inconspicuous nucleoli
Keratin pearls & intercellular bridges
Occasional mitotic figures
Infiltrative growth pattern
Large-cell nonkeratinizing squamous cell carcinoma
Large cells of similar size and shape
Moderate cytoplasm
May have individual cell keratinization
Keratin pearls are absent
Prominent nucleoli
Mitotic figures are common
Invasive edge is smooth
Histologic grade
Degree of differentiation of tumor cells
Based on amount of keratin, degree of nuclear atypia, mitotic activity
Correlates with frequency of pelvic nodal metastasis
Grade 1: Well differentiated
Abundant intercellular bridging
Cytoplasmic keratinization
Keratin pearls
Cells are uniform with minimal nuclear pleomorphism
Mitotic rate is < 2 mitotic figures per high-power field
Grade 2: Moderately differentiated
Individual cell keratinization
Moderate nuclear pleomorphism
Mitotic rate is ≤ 4 mitotic figures per high-power field
Grade 3: Poorly differentiated
Minimal evidence of squamous differentiation
Cells are immature with marked nuclear pleomorphism and scant cytoplasm
Mitotic rate is > 4 mitotic figures per high-power field
Ultrasound
Inadequate for diagnosis, staging, and surveillance for recurrence
Technically limited by body habitus, low signal-to-noise ratio, and lack of tissue characterization
CT
92% accuracy for assessment of stage IIIB-IVB disease
CT can demonstrate
Pelvic sidewall extension
Ureteral obstruction
Advanced bladder and rectal invasion
Adenopathy
Extrapelvic spread of disease
May see distention of uterine cavity with fluid/blood if tumor obstructs endocervical canal
CT can guide lymph node biopsy and radiation planning
CT has high sensitivity and specificity for detection of recurrent tumor
Soft tissue mass with variable degrees of necrosis
Cystic mass with minimal soft tissue
Limitations of CT
Limited visualization of primary tumor
Hypodense or isodense to normal cervical stroma
Tumor detection and depth of invasion difficult
Inaccurate for detection of parametrial invasion
Only 30-58% accuracy
Parametrial inflammation can mimic parametrial tumor infiltration
Paracervical ligaments and vessels may be mistaken for soft tissue strands
MR
Ideal for local cervical cancer staging
Superior soft tissue contrast
Multiplanar capability
Superior to clinical evaluation and other imaging modalities with regard to tumor characteristics that determine prognosis and stage
Tumor size
Parametrial invasion
Vaginal wall invasion
Pelvic sidewall extension
Accuracy of MR is 94% in selecting operative candidates
Compared with 76% for CT
Including MR in pre-treatment work-up significantly decreases number of procedures and invasive studies
Typical MR findings of cervical cancer
T2 hyperintense mass disrupting normal hypointense cervical stroma
Endophytic: Arises from endocervical canal
Exophytic: Arises from ectocervix and extends into vaginal vault
MR technique
T2WI best for visualization of tumor and local staging
FSE, small field of view (FOV), high resolution
Coronal oblique T2WI: Long & short (donut view) axis of cervix
Evaluation of depth of cervical stromal invasion
Evaluation of parametrial invasion
Sagittal T2WI
Depth of cervical stromal invasion
Visualization of invasion of vagina and urinary bladder
Helpful to distend vagina with gel
Axial T2WI
Parametrial invasion
Pelvic sidewall invasion
Rectal invasion
T2WI with fat saturation
Helpful if prominent paracervical venous plexus
IV contrast reportedly not helpful for depth of stromal invasion or parametrial involvement
Loss of soft tissue contrast due to enhancement of normal cervical stroma and variable tumor enhancement
May result in overestimation of tumor size
IV contrast is useful in advanced disease to evaluate
Rectal, urinary bladder, pelvic sidewall invasion
Pelvic fistulas
Recurrent/residual disease post radiation or surgery
Characteristic features of adenoma malignum
Multicystic mass extending from superficial to deep cervical wall
Mass may be nodular or annular
Mass invades deep into cervical stroma
Cystic components are hyperintense on T2WI with intervening low signal septations
Solid enhancing components help differentiate adenoma malignum from benign entities
Limitations of MR
Differentiating tumor recurrence from early radiation change and infection
May overestimate parametrial invasion with large tumors
Due to surrounding stromal edema from tumor compression or inflammation
PET/CT
Excellent for detection of lymphadenopathy and distant metastatic disease
100% sensitivity and 99.6% specificity for lymph nodes > 5 mm in short axis
100% sensitivity and 94% specificity for distant metastatic disease
PET is superior to MR and CT for depiction of adenopathy
Metabolic changes may precede morphologic changes
Moderate to marked increase FDG uptake relative to normal structures
SUV is not helpful when characterizing lymph node lesions
Limitations
Lower spatial resolution compared to CT and MR
Cannot differentiate malignant from reactive adenopathy
Cannot differentiate malignant, infectious, or inflammatory processes
Poor anatomic resolution of PET is overcome by fusion with CT
General comments
Accurate staging is critical for guiding management
Important to avoid upstaging at time of surgery
Significant increase in morbidity when surgery and radiotherapy are combined
International Federation of Gynecology and Obstetrics (FIGO)
Clinical staging of cervical cancer
Preferred staging system in order to provide uniformity
Results of imaging technologies (CT, MR, PET) should not be used to determine clinical stage
Not universally available
Can be used for prognostic information and treatment planning
Surgical and pathologic findings should not change clinical stage
Can be used in TNM staging
Clinical stage must not be changed for subsequent findings once treatment started
If there is doubt regarding stage, the lesser stage should be used
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree