Fig. 2.1
Age-adjusted SEER incidence rates for prostate cancer
Increased prostate cancer incidence was only one of several effects brought upon by widespread PSA screening. Another result of PSA screening is stage migration, a shift toward diagnosing prostate cancer at earlier stages (Fig. 2.2). In a report published in 1993, Catalona et al. found that PSA screening increased the detection of prostate cancers that were organ confined [4]. Of men diagnosed with prostate cancer, locally advanced disease was found in 57% of those screened by digital rectal examination alone but in only 29% of those screened by serial PSA measurements. Results from a study that involved 5,568 men with prostate cancer treated at the Mayo Clinic between 1987 and 1995 provided further evidence of stage migration [5]. During this period, the percentage of men with clinical stage T1c prostate cancer increased from 2.1% to 36.4 %while those with clinical stage T3 prostate cancer decreased from 23.5% to 6.5%. Other reports have confirmed significant prostate cancer stage migration due to the introduction and extensive use of PSA as a prostate cancer screening test [6–8]. Notably, the PSA threshold used as a trigger for prostate biopsy in these earlier studies was 4.0 ng/ml [9]. Subsequently, the Prostate Cancer Prevention Trial found that a significant number of men with PSA <4.0 ng/ml also harbored prostate cancer [10]. As a result, the National Comprehensive Cancer Network and the European Association of Urology recommended considering prostate biopsy in men with PSA <4.0 ng/ml [11, 12]. Also, modern transrectal biopsy techniques have increased the number of cores removed from 6 to up to 18 since multiple studies have demonstrated a high false-negative rate with traditional sextant biopsy [13–15]. The combination of lower PSA thresholds and increased number of cores removed during biopsy has led to the vast majority of detected cancers being organ confined. SEER data from 2000 to 2007 showed that 80% of prostate cancer cases diagnosed were localized while only 4% were metastatic.
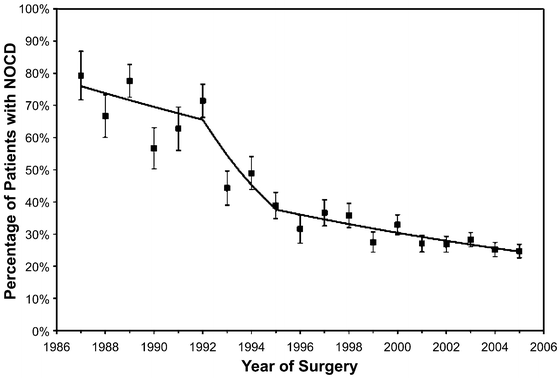

Fig. 2.2
Trends in prostate cancer pathologic stage migration. NOCD non-organ confined disease (from Dong et al. [115]; with permission)
Concomitant with stage migration is a trend toward diagnosis of increasing numbers of low-risk tumors. Cooperberg et al. examined data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) to characterize trends in the clinical presentation of prostate cancer as well as management decisions for low-risk disease [16]. Low-risk features were defined according to the D’Amico classification as clinical stage T1c, T2a, PSA level ≤10 ng/ml and Gleason score ≤6 [17]. In men diagnosed with prostate cancer between 1989 and 1992, the proportion with low-risk features was 29.8%. This increased to 45.3% in men diagnosed between 1999 and 2001.
What happens to the increasing number of men who are diagnosed with low-risk, localized disease? Han et al. reported the outcomes of 2,370 men who underwent radical retropubic prostatectomy between 1982 and 1998 [18]. Echoing other studies, the authors found a significant stage migration during the study period. In the early 1980s, <50% of men presented with organ-confined disease which increased to 70% by 1998. Biochemical recurrence-free survival (RFS) rates after surgery improved substantially over this time period. Men treated between 1982 and 1988 had a 5-year biochemical RFS rate of 77% whereas men treated between 1992 and 1998 had a 5-year biochemical RFS rate of 87%. In another study involving a multi-institutional cohort of 12,677 men who underwent radical prostatectomy between 1987 and 2005, the 15-year prostate cancer-specific mortality (PCSM) was only 12% for all risk groups [19]. For the patients who presented with clinical T1c disease, the 15-year PCSM was even lower at 6%, with all decedents displaying unfavorable characteristics such as PSA > 10 ng/ml, Gleason score ≥ 7, or disease in the lymph nodes or seminal vesicles on final pathology. Radiation therapy has been similarly effective. One group studied 381 patients who underwent external beam radiotherapy with a median dose of 70.4 Gy for clinically localized prostate cancer between 1989 and 2000 [20]. They reported an improvement in 2-year RFS from 67% to 91% between the earliest study period and the latest study period. Even taking into account the “Will Rogers phenomenon” due to Gleason score shift [21], these data clearly establish that the available radical treatment modalities are extremely effective at controlling low-risk, localized disease.
Indeed, one is naturally led to contemplate the optimum management for low-risk prostate cancers detected by PSA screening. Albertsen et al. reported 20-year outcomes for men with clinically localized prostate cancer who were managed conservatively with either observation or androgen deprivation therapy alone [22]. In this study, the mortality rate for men diagnosed with Gleason score ≤6 prostate cancer was just 18 per 1,000 person-years, compared with 121 per 1,000 person-years for men with Gleason score 8–10 prostate cancer. Further data from the US Prostate, Lung, Colorectal, and Ovarian (PLCO) Screening Trial [23] and the European Randomized Study of Screening for Prostate Cancer (ERSPC) Trial [24] cast doubt on whether immediate treatment for cancers detected by PSA screening is necessary. Results from the PLCO trial demonstrated that over a median follow-up period of 11 years, there was no mortality benefit from screening for prostate cancer with PSA and digital rectal examination on an annual basis. The ERSPC trial showed a modest reduction of 7 prostate cancer deaths per 10,000 men screened after 9 years of follow-up. Notably, the estimated number needed to screen was 1,410, and the number needed to treat was 50 to prevent one prostate cancer death. Critics argue that the PLCO trial is inherently flawed due to PSA screening in 52% of the controls and a short follow-up time that is incompatible with the rate at which prostate cancer causes death. Nonetheless, it demonstrates that there are definitely men who have indolent cancers that may not benefit from immediate treatment. Draisma et al. estimated overdiagnosis, as defined by prostate cancer discovered during PSA screening which would not have contributed to a patient’s mortality, in up to 42% of cases [25]. Additionally, lead time for cancers that would have become clinically diagnosed without screening is 5.4 to 6.9 years. These findings provide further evidence that screen-detected prostate cancers that are low stage and low grade may not harm a patient in his lifetime. Current treatment patterns, however, are not congruent with this observation. An analysis of the SEER data between 2004 and 2006 for men newly diagnosed with prostate cancer and PSA ≤4 ng/ml revealed that 54% had D’Amico low-risk disease, but over 75% of them underwent definitive therapy such as radical prostatectomy or radiation therapy [26]. As we understand more about the biology of screen-detected, low-risk prostate cancers, the notion that otherwise healthy men with this disease require aggressive treatment has been questioned. Two alternative strategies proposed for the management of screen-detected, low-risk prostate cancer include active surveillance and focal therapy.
Active Surveillance
Definition and Rationale
The goal of active surveillance is to identify men who do not have life-threatening cancers so that they can avoid the morbidity of immediate, radical treatment [27]. The rationale for active surveillance stems mainly from the two main quandaries caused by PSA screening, overdiagnosis, and lead time bias. Despite uncertainty regarding tumor behavior in this setting, most men (and their physicians) still opt for some form of active treatment, whether it is radical prostatectomy or radiation therapy. These therapies can result in significant complications. Both modalities are associated with erectile dysfunction, radical prostatectomy is associated with urinary incontinence, and radiation therapy is associated with gastrointestinal toxicity. Such treatment can negatively affect a patient’s quality of life many years later [28]. By only targeting men with significant cancers for therapy, active surveillance aims to spare men with indolent cancers from the morbidities of radical treatment. Notably, active surveillance must be distinguished from watchful waiting. The latter refers to initiating palliative treatment for a prostate cancer only after it has become symptomatic. This strategy is typically employed for patients with a short life expectancy and likely to die from a competing cause.
Patient Selection
The ideal prostate cancer patient for active surveillance is a man diagnosed with organ-confined, low-risk prostate cancer who will be compliant with a surveillance protocol and can accept a small risk that his cancer may spread beyond his prostate despite using modern biopsy techniques and regular monitoring. Stamey et al. performed one of the initial studies on defining clinically insignificant prostate cancer [29]. The authors demonstrated, through the examination of 139 cystoprostatectomy specimens from patients with bladder cancers, that prostate cancers <0.5 cc are unlikely to reach clinical significance during the patient’s lifetime. Epstein et al. subsequently developed a biopsy- and PSA-based model to predict whether a prostate cancer would be <0.5 cc if removed by radical prostatectomy [30]. These criteria include Gleason score ≤6 (no pattern 4 or 5 disease), tumor in no more than 2 cores from a sextant biopsy, no core involved by more than 50% tumor, and PSA density ≤0.15 ng/ml. The diagnostic accuracy was 73%. If a man undergoing surgery had any adverse findings on biopsy or PSA density >0.15 ng/ml, there was an 83% probability that the tumor was larger than 0.5 cc or not organ confined. These findings have led some investigators to adopt Epstein’s criteria for identifying patients suitable for active surveillance. When these criteria, in addition to PSA < 10 ng/ml, were applied to 1,886 prostate cancer patients from the CaPSURE database diagnosed between 1999 and 2004, 16.4% were eligible for active surveillance [31]. Nomograms have also been developed to predict the presence of indolent prostate cancer, but they have not been utilized in any current published active surveillance studies [32, 33].
A study performed at Cleveland Clinic examined the ability of Epstein’s criteria to predict for insignificant disease, organ-confined disease, and biochemical RFS [34]. The authors utilized two definitions for insignificant disease. The classical definition was organ-confined cancer, Gleason score ≤6, and tumor volume <0.5 cc. The more liberal definition was organ confined and Gleason score ≤6 tumor of any volume. Of men who met Epstein’s criteria, 92% had organ-confined disease, and the estimated 5-year biochemical RFS was 100%. These data suggest that Epstein’s criteria predict for organ-confined disease and a high likelihood of cure by radical prostatectomy. However, analysis of the radical prostatectomy specimens showed that a substantial percentage of men who met Epstein’s criteria had foci of Gleason 7 disease and extraprostatic extension. Thus, patients should be advised that use of these criteria does not exclude the presence of a significant prostate cancer (i.e., Gleason ≥ 7 and/or non-organ confined) either at diagnosis or during active surveillance. Conti et al. presented data that correlated different active surveillance inclusion criteria with rates of adverse pathological features such as Gleason upgrade, extracapsular extension, and seminal vesicle involvement [35]. Fewer men met the more stringent criteria, but they also had lower rates of seminal vesicle invasion and extraprostatic extension. In light of this discussion regarding selection criteria for active surveillance, some investigators have chosen more relaxed conditions such as allowing men with Gleason pattern 4 to be observed [36–38]. It is anticipated that less stringent criteria would decrease the number of organ-confined cancers [39], and their use in men initially choosing active surveillance and expecting a high likelihood of cure by radical treatment may lead to disappointment. Life expectancy and comorbidities should be taken into account when considering the use of more relaxed criteria.
Active Surveillance Protocols
There is currently no consensus regarding an optimum active surveillance protocol or trigger for intervention. Nonetheless, investigators have used serial digital rectal examinations, PSA measurements, and prostate biopsies to monitor patients. Other tests considered include percent-free PSA measurement and transrectal ultrasound. The intervals between tests vary widely. For example, men enrolled in Carter et al.’s study underwent measurements of total and free PSA on a semiannual basis [40] while other groups elected to perform PSA measurements every 3 months [41, 42].
These differences are related to the choice of trigger for intervention. Changes in PSA kinetics did not lead to a decision to intervene in Carter et al.’s active surveillance cohort, since the authors believe that, by the time changes in PSA doubling time suggest progression, the opportunity for cure may have been lost [40]. Instead, development of adverse features on biopsy was the main reason for switching to radical treatment. The justification for incorporating PSA kinetics as a trigger for intervention in an active surveillance cohort comes from multiple reports showing a correlation between increased changes in PSA over time and prostate cancer-specific mortality. D’Amico et al. showed that a PSA velocity >2.0 ng/ml per year is associated with a significantly shorter time to death from prostate cancer [43, 44]. Carter et al. found that PSA velocity 10–15 years prior to prostate cancer diagnosis, when PSA is typically under 4.0 ng/ml, is correlated with risk of prostate cancer death [45]. Cancer-specific survival was 92% in men with PSA velocity ≤0.35 ng/ml per year but only 54% in men with PSA velocity >0.35 ng/ml per year. Data from these reports suggest that PSA velocity can help identify lethal prostate cancer. Certainly, this is why some investigators use PSA velocity as a selection criterion for active surveillance as well as a trigger for intervention. Nevertheless, an evidence-based PSA velocity cutoff for either of these applications has not been established. Dall’Era et al. chose an increase in PSA velocity of >0.75 ng/ml per year as one definition for progression [46]. However, it is important to recognize that most men with newly diagnosed disease do not have PSA velocities that approach this value, especially when PSA is low. Data from the Baltimore Longitudinal Study of Aging showed that in a population that underwent PSA screening, the median PSA velocity for those with cancer were 0.08 ng/ml per year and 0.50 ng/ml per year for men with PSA < 3 ng/ml and 3–10 ng/ml, respectively [47]. Klotz et al. initially used PSA doubling time of 2 years as a trigger [42]. This value was recognized as overly stringent since it classified only 10% of patients as high risk, so the authors chose to increase the cutoff to 3 years. One must also use caution in the method used to calculate PSA velocity, as the interval between measurements can severely affect the results [48]. Additionally, PSA doubling time and PSA velocity may not be equivalent in identifying those with life-threatening prostate cancer [49].
Neither PSA kinetics nor adverse biopsy findings is a perfect trigger for radical treatment, but there is some evidence that PSA kinetics should not be used alone. Men enrolled in Carter et al.’s study received annual biopsies and provided an opportunity to examine if PSA kinetics is a good predictor of adverse pathology on surveillance biopsy. Ross et al. found that PSA doubling time was not significantly associated with subsequent adverse findings, whereas PSA velocity was marginally significant (p = 0.06) [50]. Importantly, the area under the ROC curve (AUC) for both values were approximately 0.60, and neither was associated with adverse pathology in men from this cohort who underwent radical prostatectomy. These data suggest that annual surveillance biopsy should not be replaced by PSA kinetics.
The presence of adverse features such as increased Gleason score on subsequent biopsies is a less controversial trigger for intervention. Changes in histology that result in recommendation for radical treatment occur in approximately 30–40% of treated participants [40–42, 46]. Of note, when upgrading is noted, it is important to take into account repeat biopsy timing. Berglund et al. examined patients eligible for active surveillance based on modified Epstein’s criteria and PSA < 10 ng/ml [51]. They underwent repeat 14 core biopsy (standard 12 core biopsy with 2 cores directed at the transition zone) within 3 months of a first biopsy, and 27% were upgraded and/or upstaged. These results imply that undersampling is an issue and underscore the need for a systematic and thorough biopsy scheme to determine eligibility prior to enrollment into an active surveillance program. A report by Epstein et al. examined the interval to grade change on repeat biopsy [52]. In their series, 12.9% of men had upgrading on repeat biopsy. Of these men, 89% had a grade change by 15 months, and only 4% had a grade change at 24 months or greater. The authors emphasized that men with no grade change were more likely to undergo more follow-up biopsies, thus eliminating undersampling as a possible cause for this observation. Taken together, the data demonstrate that inadequate tumor sampling is responsible for early upgrading and that prostate cancer grade is unlikely to increase significantly over the course of 2 years. Thus, most active surveillance protocols recommend repeat biopsy more than 1 year from the initial biopsy provided that the initial biopsy was adequate and there is no worrisome change in other clinical data such as PSA velocity or digital rectal examination findings.
Outcomes
Outcomes from contemporary series of active surveillance are promising (Table 2.1) [37, 40–42, 46, 53–55]. Despite the heterogeneity with regard to inclusion criteria and triggers for intervention, cancer-specific survival remains between 97 and 100%. These results, however, are tempered by the relatively short median follow-up across all studies. Klotz et al. have, so far, reported the longest median follow-up of 6.8 years. In their series, there were 5 deaths due to prostate cancer. Out of 450 men, only one who was subjected to an observation period >2 years and subsequently treated died of prostate cancer. However, the 5-year biochemical RFS rate was only 47% for the 117 patients treated with surgery or radiation. This unexpectedly low biochemical RFS rate is unlikely due to poor patient selection at time of trial enrollment, because, of the 85 patients who were intermediate risk at baseline, only 1 experienced progression to metastasis and death. The authors did find that men with PSA doubling time <3 years prior to treatment were 8.5 times more likely to experience biochemical failure. If the entire cohort were to be used as the denominator, that is, if every man was subjected to radical therapy, the actual 5-year biochemical RFS rate is 13%. This is still significantly higher than the nomogram predicted 5-year biochemical RFS rate for low-risk patients undergoing radical prostatectomy [56]. Duffield et al. examined findings at prostatectomy for men who had disease progression on active surveillance [57]. After initial diagnosis, the mean time to prostatectomy was 29.5 months. The investigators found that 27% of tumors were potentially insignificant, and 35% were not organ confined. In van den Bergh et al.’s study, immediate prostatectomy was compared with prostatectomy delayed a median of 1.8 years after diagnosis [58]. Although there were few patients in both groups and differences in pathologic characteristics at prostatectomy were not statistically significant, there was a trend toward more adverse features in the delayed prostatectomy group. For example, delayed prostatectomy was associated with a 2.5 times higher odds for capsular penetration. Taken together, these reports suggest that there is some increased risk of adverse pathologic features when treatment is delayed. In contrast, Dall’Era et al. presented data showing men with low-risk prostate cancer who underwent active surveillance for a median of 18 months prior to radical prostatectomy had similar rates of Gleason upgrading to ≥ 7, pathologic stage T3, and positive surgical margins when compared with men who underwent immediate surgery [59]. Long-term oncologic outcome studies will be essential in comparing cancer control between immediate prostatectomy and delayed prostatectomy after a period of active surveillance. In summary, the triggers for intervention for men on active surveillance will need to be investigated more thoroughly so that there is an appropriate balance between the number of interventions and the number of men who may die of prostate cancer while on active surveillance. Establishing an optimal trigger will be especially important for men with a long life expectancy who are considering active surveillance. In spite of these reservations, the current trials certainly support that active surveillance is a safe alternative to radical therapy for low-risk, localized prostate cancer. Recently, Hayes et al. performed a decision analysis which showed that active surveillance was associated with the highest quality adjusted life years (QALY) when compared with radical treatment [60]. Of note, the difference in quality adjusted life expectancy between active surveillance and radical prostatectomy was only 10 months (11.07 vs. 10.23 QALYs). Certainly, there are a number of men who believe that the high likelihood of cure with radical prostatectomy is worth the potential negative side effects.
Table 2.1
Summary of outcomes for active surveillance
Authors | Patients (n) | Median age (years) | Median follow-up (months) | Inclusion criteria | Triggers for intervention | Cancer-specific survival | % Remaining on AS |
|---|---|---|---|---|---|---|---|
Carter [40] | 407 | 66 | 41 | Epstein criteria | Adverse pathologic features on repeat biopsy | 100 | 59 |
van As [54] | 326 | 67 | 22 | PSA ≤ 15 ng/ml Gleason ≤ 3 + 4 <50% cores (+) <10 mm any core | PSAV > 1 ng/ml per year Gleason ≥ 4 + 3 > 50% cores (+) | 100 | 73 |
Khatami [53] | 270 | 64 | 63 | <3 cores (+) <2 mm per core | Progression in PSA, stage, or grade | 100 | 61 |
Roemeling [37] | 278 | 70 | 41 | T1c or T2 PSA ≤ 15 ng/ml Gleason ≤ 8 | Progression in PSA | 100 | 71 |
Dall’Era [46] | 321 | 64 | 43 | T1 or T2a PSA < 10 ng/ml Gleason ≤ 6 <33% cores (+) | PSAV increase of >0.75 ng/ml/year | 100 | 76 |
Eggener [41] | 262 | 69 | 29 | Age <75 years T1 or T2a PSA ≤ 10 ng/ml Gleason ≤ 6 ≤3 cores (+) | Progression in PSA, stage, grade, or MRI findings | 100 | 84 |
van den Bergh [55] | 533 | 70 | 48 | T1c or T2 PSA ≤ 10 ng/ml PSAD < 0.2 ng/ml per ml Gleason ≤ 6 ≤2 cores (+) | PSA DT < 3 years | 99 | 50 |
Klotz [42] | 450 | 70 | 81 | Gleason ≤ 6 PSA ≤ 10 ng/ml | PSA DT < 2 years (prior to 1999), <3 years (after 1999) | 97 | 53 |
Total | 2,849 | 64–70 | 22–81 | – | – | 97–100 | 50–84 |
One of the main concerns regarding active surveillance is the dropout rate. Between 50 and 84% of men remained on active surveillance in these studies, but up to 50% of men who chose radical treatment after an initial period of active surveillance did so despite lack of evidence for disease progression [61, 62]. Anxiety regarding a cancer diagnosis and prognostic uncertainty due to the heterogeneous biology of prostate cancer have often been cited as motivating factors for a patient to choose radical treatment. Using a questionnaire to measure cancer anxiety in men on active surveillance, Latini et al. found that the rate at which cancer anxiety changed was modestly associated with treatment receipt [63]. Litwin et al. examined longitudinal changes in the RAND 36-Item Health Survey for men choosing radical prostatectomy, radiation therapy, or watchful waiting [64]. Although the three groups appeared similar at early time points, men who chose watchful waiting developed significantly lower scores after 24 months in mental health, social function, role limitations due to emotional problems, and vitality when compared with those who chose radical prostatectomy. One explanation for this observation is that the watchful waiting group was already disadvantaged due to higher number of comorbidities and lower general health perception. Nevertheless, one cannot minimize the effects of anxiety, worry, and depression in these men. Another study found that, in men choosing active surveillance, anxiety and distress remained low after 9 months of surveillance [65]. However, one possible explanation is that patients who decide on active surveillance as a management strategy may already have lower anxiety and distress about their disease than those who choose radical treatment. Additionally, clinical data regarding whether prostate cancer has progressed may be lacking at the end of 9 months, so patients may not have had the information to react.
Approaches to lower anxiety during active surveillance should include fully educating the patient and ensuring that he understands that active surveillance is legitimate and widely accepted for low risk, localized prostate cancer. The American Urological Association, European Association of Urology, and National Comprehensive Cancer Network all consider active surveillance as a viable option, and this should be emphasized to patients. Professional psychosocial support between physician visits should be strongly considered, and there should be every effort to engage the patient’s family and social support network. With these elements in place, the chances that a man chooses radical treatment despite lack of measurable progression should decrease.
Focal Therapy
Goal and Rationale
As discussed previously, PSA screening and the heterogeneity of prostate cancer biology has led to overuse of radical treatment for this disease with resulting lifelong side effects. Focal therapy attempts to take advantage of the lead time bias afforded by PSA screening by destroying known areas of cancer while still organ confined and sparing uninvolved tissue so that urinary continence and potency are maintained. For focal therapy to be successful, several assumptions are made, some of which have good data to support while others require more investigation.
Prostate cancer is a multifocal disease in up to 87% of cases [66]. This observation alone raises questions regarding the potential efficacy of focal therapy. Nonetheless, there is increasing evidence that an index lesion drives prostate cancer biology and clinical outcome. Wise et al. examined the distribution of tumor volumes and rates of biochemical recurrence in men with multifocal disease who underwent radical prostatectomy in the PSA era [67]. They found that the mean index tumor volume was 4.2 cc, approximately 6.6 times the size of smaller cancer foci. In each prostate, there was a median of 2 additional smaller cancer foci discovered at prostatectomy, and 58% of these lesions were ≤0.5 cc. Importantly, the index cancer volume and total cancer volume were equally predictive of biochemical recurrence, suggesting that smaller foci alone are not a significant predictor of clinical outcome. Noguchi et al. studied the characteristics of secondary cancers and their relationship to biochemical recurrence in 222 men who underwent radical prostatectomy for T1c prostate cancer [68]. Cancers were classified into three groups. There was a single tumor in 24% of cases, an index tumor with secondary cancers <0.5 cc in 39% of cases, and an index tumor with secondary cancers more than 0.5 cc in 37% of cases. There was no difference in preoperative PSA, number of positive cores, or percent Gleason pattern 4/5 cancer among these groups. However, the multifocal group with secondary cancers <0.5 cc had a better outcome than the unifocal group. These data imply that controlling the index lesion may be sufficient in adequately treating prostate cancer. Other studies also confirm this relationship between the index lesion and secondary cancers [69, 70]. One important report used genome wide single nucleotide polymorphism and copy number analysis to examine the relationship between multiple metastatic sites from men who died from prostate cancer [71]. The authors provide compelling evidence that lethal metastatic deposits are typically derived from a common parental clone, and genomic changes resulting in subclones are sustained throughout disease progression. One drawback of the study is that only 5 of the 30 men had tissue from the primary site available for analysis thus limiting any relationship that can be drawn between metastatic sites and the index lesion. Nevertheless, the authors found no difference in copy number patterns between the primary site and metastatic deposits in these individuals. These findings strengthen the concept that multifocality is not a barrier to focal therapy as long as the lesion that will ultimately cause morbidity and mortality can be identified and treated.
Identifying and Characterizing the Index Lesion
Identifying the index lesion and determining its most critical characteristics for surgical planning is perhaps one of the most challenging aspects of implementing focal therapy. Biopsy and magnetic resonance imaging (MRI) are the modalities recommended by an international panel for determining if a lesion is amenable to focal therapy [72]. Although extended scheme biopsy has an incremental advantage over sextant biopsy in determining whether a prostate cancer is unilateral, the diagnostic accuracy is only 59% [73]. Thus, there is still a significant probability of cancer on the contralateral side even if biopsy only detected unilateral cancer. Yoon et al. studied 100 radical prostatectomy specimens from which initial biopsy indicated limited, unilateral disease (<3 positive cores, <50% of each core involved with cancer, Gleason score 6 or less) [74]. Additionally, there was only one positive core in 66 cases. Concerning findings from this report include 65% of cases having tumor on the contralateral side, 23% of cases having larger tumors on the contralateral side, and 14% of cases having either Gleason pattern 4 or extraprostatic extension on the contralateral side. Therefore, transrectal biopsy, as currently performed, is not only poor at determining unifocal versus multifocal disease but also can fail to identify significant, adverse pathology leading to inadequate treatment by focal therapy. Moreover, grade and extent of cancer correlate very poorly between biopsy and radical prostatectomy specimen [75]. Thus, it is questionable whether transrectal biopsy alone can accurately identify the index lesion for focal therapy planning.
Due to these concerns, MRI has been promoted as a complementary modality in characterizing the lesion initially found on biopsy by providing information such as size and location. Other roles of MRI in this setting include ruling out locally advanced cancer and enlarged pelvic lymph nodes suggesting metastasis. Multiple studies have shown that endorectal MRI findings can improve accuracy in predicting whether or not prostate cancer is truly organ confined. For example, Wang et al. studied MRI findings in 229 patients and combined MRI-MR spectroscopy findings in 383 patients who underwent radical prostatectomy [76]. They found that use of MR findings for the low-risk cancer group improved the AUC of the 2001 Partin tables from 0.60 to 0.78. The same group demonstrated that endorectal MRI can also be useful in predicting seminal vesical invasion [77]. Furthermore, Jeong et al. studied 130 patients who underwent extended scheme biopsy and MRI prior to prostatectomy and asked whether these modalities can successfully predict unilateral prostate cancer [78]. The authors found that, although extended scheme biopsy was extremely poor at predicting unilaterality, a combination of T2-weighted and diffusion-weighted MRI performed reasonably well with AUC = 0.81. Thus, although current MRI technology is not perfect, it should be used as an adjunct to biopsy in determining whether a cancer is unilateral and organ confined. These studies still do not answer whether MRI was capable of identifying the index lesion.
Tumor burden is another very important parameter for focal therapy planning. Since prostate cancers >0.5 cc in volume can be clinically significant, MRI needs to be able to detect all tumors above this volume. Older literature that employed mainly T2-weighted MRI alone reported significant difficulty detecting tumors smaller than 1 cm in diameter, corresponding to a volume of 0.53 cc if the cancer’s shape is assumed to be spherical. More than 80% of tumors larger than 1 cm in diameter were detected [79, 80]. Recent studies using dynamic contrast-enhanced (DCE) MRI were able to detect foci >0.5 cc with 80–85% sensitivity [81, 82




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree