Fig. 10.1
Comparison of dose-distribution curves of photons and protons. The high entrance dose and tail of exiting dose are avoided by the use of charged particles
Stereotactic radiosurgery (SRS) and charged particle radiotherapy are not mutually exclusive. SRS is conceptually defined as the delivery of large, hypofractionated doses of radiation to precise targets. This definition is not limited to any particular radiation modality, and charged particles can be used in place of photons for stereotactic treatment, provided that the essential processes of SRS—including accurate dose-modeling, image-guidance, and quality assurance—can be achieved. In fact, combing the radiobiological and dose escalation advantages of hypofractionation with the tissue-sparing characteristics of charged particle radiation can be an elegant way to maximize the therapeutic index.
Until 2001, there were only two proton therapy centers in the USA. Since then, the attractive properties of charged particles have encouraged the construction of eight additional centers with four more to be completed soon. This sudden acceleration in proton center availability will likely fuel active investigation into the use of charged particle radiation in a variety of clinical settings, including radiosurgery. This chapter will review the physics and radiobiology of charged particle radiotherapy as well as published clinical outcomes to date in the hypofractionated, stereotactic setting.
Physical and Technical Considerations
For therapeutic radiation, charged particles are first produced by applying an electric field to a gas of the desired particle to separate the nucleus from its electrons. Acceleration of the nucleus-derived charged particle is then achieved via a cyclotron or a synchrotron. These machines operate by accelerating particles around a round cavity while subjecting them to electric and magnetic fields. In a synchrotron, both the electric field and the magnetic field vary in time to accelerate a particle while also keeping it along a defined circular track as it accelerates. In a cyclotron, the particle starts in the center of a cylindrical cavity and accelerates in a spiral, moving centripetally with time. In this case, the electric field alternates with a constant frequency to accelerate the particle during each revolution, but the magnetic field is kept uniform to achieve the spiral movement.
When the particle achieves the desired energy, it is ejected from the synchrotron or cyclotron. In the case of protons, this is usually 150–250 MeV which results in 25–30 cm of penetration into tissue. After ejection from the accelerator, the particle is then guided by magnetic fields to the treatment gantry. A nozzle at the gantry directs the beam to the properly positioned and immobilized patient. Worldwide, proton and carbon ion cyclotrons are the most commonly used systems for charged particle therapy. Other charged ions including helium, neon, argon, and silicon nuclei have been explored in research settings but are not currently in widespread use.
A monoenergetic beam of charged particles results in a narrow Bragg peak, which has limited clinical utility for treating typical tumor widths. Therefore, two different modes of delivery have been developed to adequately cover the dimensions of a typical target. The most common method of delivery is via passive scattering. In this system, the particle beam is scattered and flattened to create a beam of desired width and intensity. The monoenergetic beam can also be modulated with a range shifter to generate a polyenergetic beam; the resulting depth–dose curve of the polyenergetic beam is a superposition of the depth–dose curves of the constituent Bragg peaks. It is characterized by a nonzero entrance dose which rises to a plateau region before falling to zero at the depth of the most distal constituent Bragg peak (Fig. 10.2). The range shifter can customize the width of this “spread-out Bragg peak” so that the tumor is adequately covered by the high-dose plateau.
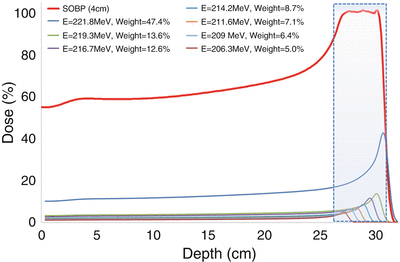

Fig. 10.2
Depth–dose curve for a spread-out Bragg peak (SOBP) with its constituent pristine Bragg peaks also shown. The gray area representing the target is encompassed by the width of the SOBP plateau region
To maximize conformality to the target’s three-dimensional shape and to avoid normal tissues, additional shaping of the radiation field in passive scattering systems is usually necessary. Either apertures or collimators are used to shape the perimeter of the beam, while customized compensators placed in the beam path after the aperture can be used to finely modulate the field’s distal edge [1, 2]. Importantly, the materials used for these devices must provide adequate attenuation without producing harmful particles through scatter interactions. For example in the case of proton therapy, brass or tungsten apertures are often used.
Because passive scattering planning is similar to 3D conformal radiation, complicated dose distributions and tight conformation of dose around curved lesions are difficult to achieve. However, with proper resources and skilled dosimetry, these challenges are usually surmountable. Dosimetric studies of passive scattering proton therapy in the thorax have shown that, with proper planning, their dose distributions are superior to photon-based treatments (3D conformal photon or photon IMRT) in most cases [1, 3].
The second mode of delivery is a scanning beam system. In this mode, the charged particle is subjected to an electromagnetic field prior to exiting the gantry. This electromagnetic field modulates the energy and direction of the particle beam so that the particles exiting the gantry deposit their energy at a specific plane (“uniform scanning”) or voxel (“spot scanning” or “pencil beam scanning”) within the tumor [4]. In these systems, all the planes or voxels that constitute the target are treated in turn, usually starting at the most distal areas, until the entire volume of the tumor has been irradiated. Among the voxel-based modes, spot scanning delivers dose to each voxel in a step-and-shoot fashion while pencil beam scans and treats each voxel in a continuous fashion. In both cases, the dose that each voxel receives can be independently modulated, and therefore treatment planning for these techniques is typically done using inverse-planning algorithms. Because of this similarity with photon-based intensity-modulated radiotherapy (IMRT), these techniques are often called intensity-modulated proton therapy (IMPT).
Scanning beam delivery has two important advantages. First, compensators and apertures are not needed, and therefore, contaminant scatter from these objects and the risk of secondary malignancy is reduced. Second, scanning modes result in greater conformality and are especially useful for eccentric tumors or tumors located near sensitive structures. A disadvantage, on the other hand, is that this approach is less forgiving when intra- and interfraction tumor and organ motion occurs [5–9]. To address intrafractional motion, it is imperative that tumor motion be accounted for during simulation, treatment planning, and dose delivery. In the chest, for instance, tumors’ position can change significantly over the course of respiration. Investigators at MD Anderson have demonstrated that the creation of a four-dimensional, internal gross tumor volume (“4D-iGTV”) based upon the maximal intensity projection of the tumor on axial CT slices throughout the entire respiratory phase is an accurate method for minimizing the risk of a geometrical miss due to tumor motion [10]. For interfraction changes due to an early tumor response, adaptive planning can be considered [8, 9].
Radiobiologic Advantages of Charged Particle Therapy
Charged particle radiotherapy is directly ionizing because, by virtue of their charge, the accelerated particles can interact with atomic electrons through Coulombic forces. Consequently, charged particles, unlike photons, are associated with high linear energy transfer (LET). Their interaction with tissues is characterized by (1) direct damage to cellular DNA and (2) a dense mobilization of secondary electrons along the particle track, resulting in a high local concentration of free radicals which also cause DNA damage. In clinical systems that utilize a SOBP, cellular damage is considered to be nearly constant across the plateau phase of the depth–dose curve, but in reality, there may be variations over the course of the particle track. This is discussed in more detail in section “The Challenges of Particle Therapy”.
Though different nuclei used in charged particle therapy may have similarities with respect to the general shape of their depth–dose curve, their ultimate radiobiological effects is dependent upon the specific LET of the particle that is used. Said differently, the shape of the SOBP is useful for determining the distribution of dose, but the extent of biological damage that is done to the target tumor is dependent on a different property: relative biological effectiveness (RBE). This quantity is defined as the ratio of the dose of photons to the dose of charged particles necessary to achieve the same biological effect in a specified test system. If the RBE for a specific particle is high, then that particle exerts a significant amount of damage per absorbed unit of energy and is said to have high “quality.”
RBE is related to LET in a bell-shaped distribution: RBE rises until an LET of approximately 100–150 keV/μm and then falls at higher LET values. The reason for the peak in RBE at 100 keV/μm is that the likelihood of a single-particle track causing a DNA double-strand break is maximized at this level. Because each charged particle has a different LET, we expect RBE to be different among them. For instance, protons in the 250 MeV range have an RBE of approximately 1.1, whereas carbon-ions have an RBE in the 2–3 range [11, 12]. This means that protons are radiobiologically similar to photons, offering a 10 % improvement in radiation quality, whereas heavy nuclei cause considerably higher biological effect.
Two other radiobiological phenomena deserve mention with regard to the relative advantages of charged particle radiation. In photon radiobiology, the radiosensitivity of a tumor cell can be diminished by the absence of oxygen or by the cell’s position in a radioresistant phase of the cell cycle (such as the S phase). In both cases, the altered radiosensitivity is attributable to the cell’s capacity for repair of radiation-induced DNA damage, which is enhanced by hypoxia and the presence of sister chromatids and DNA-repair enzymes in certain cell-cycle phases. However, the single-track, double-strand break described in the previous paragraph is a subtype of DNA injury that is difficult to repair even in the presence of these radiobiological modifiers. Because high quality particles (such as heavy nuclei such as carbon ions) are more likely to induce this type of injury, the oxygen and cell cycle effects become less important. This ability of heavy ion particle radiation to overcome these intrinsic mechanisms of radioresistance is a major theoretical advantage over photons. In fact, this difference in radiobiology is the main distinguishing feature between heavy nuclei ions and protons, whose radiological quality is similar to photons.
Particle Radiosurgery: Early Clinical Outcomes
As stated in section “Introduction: The Rationale for Charged Particle Radiation for SRS”, there is no a priori reason that stereotactic hypofractionation cannot be combined with particle radiotherapy so that patients can benefit from the advantages of both. However, highly reproducible patient immobilization devices (such as the Gamma Knife frame) and image-guidance technologies (such as cone-beam computed tomography) have not been traditionally been available in proton or heavy-nuclei gantry systems. Fortunately, newer systems have circumvented these limitations by incorporating customized vacuum molded masks and frames for immobilization, four-dimensional simulation for treatment planning, and on-board imaging systems such as fluoroscopy, CT imaging, and fiducials for image-guidance. Nonetheless, “particle radiosurgery,” as loosely defined by the combination of charged particle radiotherapy with large fraction sizes, is still a young concept whose preliminary evidence is limited to a handful of single-institution series in the context of treating early-stage lung cancer. Still, despite their limitations, these experiences provide proof of principle of the efficacy and organ-sparing characteristics of particle radiosurgery.
Non-small Cell Lung Cancer
Rationale for Particle Therapy in the Thorax: Anatomy and Dose
SRS advances for non-small cell lung cancer in the last decade have largely relied on photon techniques. These early experiences were successful in safely ablating peripheral tumors but also resulted in considerable toxicity when used for centrally located tumors [13–17]. Life-threatening complications including radiation pneumonitis, great vessel rupture, esophageal ulceration, and spinal cord myelopathy have been reported when central tumors have been treated with ablative dose regimens [18, 19]. This difference in therapeutic ratio was especially stark in an early phase II study of 70 patients with both peripherally and centrally located tumors treated to 60–66 Gy in three fractions [20]. In this cohort, 2-year freedom from severe toxicity was 83 % for those with peripheral tumors compared with only 54 % for patients with perihilar or central tumors.
These anatomically challenging cases are one circumstance where particle-based radiotherapy may provide valuable synergy to hypofractionated treatment. Provided that proper beam angles are used, particles such as protons and carbon ions should significantly reduce the integral dose to the lung and minimize incidental irradiation of nearby organs including the heart, esophagus, spinal cord, bronchial tree, major vessels and brachial plexus (Fig. 10.3). Providing evidence of this principle, dosimetric studies at the Mayo Clinic and MD Anderson Cancer Center have confirmed that the Bragg peak characteristics of proton therapy can be used to deliver ablative doses of radiation to early stage tumors as effectively as photon SRS while significantly reducing the dose to surrounding normal tissues.


Fig. 10.3
Comparison of passively scattered proton therapy (right panel) and 3D conformal photon therapy (left panel)-based stereotactic ablation of a lung tumor close to critical structure brachial plexus. The proton plan results in a diminished dose to the brachial plexus
In the MD Anderson study, proton plans compared to photon-based SBRT plans significantly reduced the mean total lung dose from 5.4 to 3.5 Gy (p < 0.001) and 2.8 Gy (p < 0.001) and reduced the total lung volume receiving 5, 10, and 20 Gy (p < 0.001). Moreover, for cases where the PTV was within 2 cm of the critical structures, the proton plans resulting in significantly reduced mean maximal dose to the aorta, brachial plexus, heart, pulmonary vessels, and spinal cord [21]. Similarly, the Mayo Clinic study found that proton-based techniques resulted in significantly reduced total lung mean and V5 dose; other normal tissues including heart, bronchial tree (including trachea), esophagus, and spinal cord lower were also found to receive less dose with protons [22]. Interestingly, in both studies, better dose distributions were achieved with scanning beam methods than with passively scattered protons.
A second rationale for particle radiotherapy is the converse of the first: While the goal described above is to minimize normal tissue effects given a specified target dose, the other potential use of particle therapy is to escalate the dose delivered to the tumor given prespecified thresholds for adjacent organs. In radiosurgery for non-small cell lung cancer, this second rationale is particularly relevant because of the observation that higher BED is more likely to achieve cure [13–16, 23–32]. This phenomenon was underlined in a retrospective study by Onishi and colleagues who demonstrated that among different photon SRS regimens, patients who received a BED greater than 100 Gy had much better 5-year local control (92 % vs. 74 %) and overall survival (88 % vs. 69 %) than those who received a lower BED [23]. In light of these findings, particles may be a powerful tool for achieving 100 Gy BED when adjacent organs-at-risk prevent dose escalation of photons to these levels.
Outcomes for Highly Hypofractionated Regimens
Thus far, seven experiences using hypofractionated particle therapy for non-small cell lung cancer have been reported, four with proton therapy and three with carbon ion therapy (Table 10.1). Each of these studies has unique technical and image-guidance procedures. The earliest study was performed by Bush et al. at Loma Linda. At this center, 68 patients were treated to 51–60 cobalt-60 Gray equivalents (CGE) in ten fractions. Real-time fluoroscopy was used for verification of patient and tumor position, and notably, this study did not include any compensatory actions for respiratory motion [33]. Nonetheless, 3-year local control and cause-specific survival rates were 74 % and 72 %, respectively, over a median follow-up of 30 months. With regard to toxicity, no cases of acute radiation pneumonitis were observed nor were there any grade 3 early or late esophageal or cardiac toxicities.
Table 10.1
Highly hypofractionated proton or carbon ion for T1-T2 N0 non-small cell lung cancer
Study | Dose (CGE)/no. of fractions | Local control | Overall survival |
|---|---|---|---|
Proton therapy | |||
Westover et al. [37] (2012) | 42–50/3–5 | 100 % (2 years) | 64 % (2 years) |
Iwata et al. [36] (2010) | 80/20 or 60/10 | 81 % (3 years) | 73 % (3 years) |
Hata et al. [35] (2007) | 50–60/10 | 95 % (2 years) | 74 % (2 years) |
Nihei et al. [34] (2006) | 70–94/20 | 80 % (2 years) | 84 % (2 years) |
Bush et al. [33] (2004) | 60/10 | 74 % (3 years) | 44 % (3 years) |
Carbon ion therapy | |||
Iwata et al. [36] (2010) | 52.8/4 | 86 % (2 years) | 87 % (2 years) |
Miyamoto et al. [39] (2007) | 52.8–60/4 | 98 % (3 years) | 78 % (3 years) |
Miyamoto et al. [38] (2007) | 72/8 | 95 % (5 years) | 50 % (5 years) |
Nihei and colleagues used three-dimensional CT simulation, respiratory gating, and real-time digital radiographs for position verification. With these more modern techniques, 37 patients were treated to a total dose of 70–94 CGE using fraction sizes of 3.5–4.7 CGE, which resulted in a local control rate of 80 % at 24 months [34]. Hata and colleagues in Japan utilized 50 or 60 CGE in ten fractions to treat a cohort of 21 patients with stage I NSCLC [35]. All but one of the patients (95 %) experienced durable local control following this treatment. Two-year overall and cause-specific survival rates were 74 and 86 %. As in the Bush study, the toxicity profile was favorable with no grade 3 or greater reactions observed. Other hypofractionated proton regimens used in Japanese proton centers have demonstrated similar results [36].
The Massachusetts General Hospital reported outcomes for 15 patients with 20 tumors treated with proton SBRT to 42–50 CGE in 3–5 fractions between July 2008 and September 2010. Only one grade 3 or higher toxicity was observed (grade 3 pneumonitis in a patient with severe chronic obstructive pulmonary disease), and the 2-year overall survival and local control rates were 64 % and 100 %, respectively, with a median follow-up of 24.1 months [37].
The National Institute of Radiological Sciences (NIRS) in Chiba, Japan, has reported three trials of hypofractionated radiotherapy using carbon ions. The first, a phase I/II study of 50 patients treated to 72 CGE in nine fractions, resulted in a 5-year local control rate of 95 % with no grade 3 toxicities observed [38]. A second study followed 76 patients with T1 and T2 tumors treated to 52.8 CGE in four fractions and 60 CGE in four fractions, respectively [39]. At 3-year follow-up, local control for T1 tumors was 98 % and for T2 tumors was 80 %. Again, no grade 3 toxicities were observed. Finally, a dose escalation study in which patients receive single-fraction regimens from 28 to 44 CGE is ongoing at NIRS. Preliminary results showed no grade 3 or greater toxicities at any of the dose levels during a follow-up period of 16 months [40]. The local control and survival outcomes of this cohort are yet to be reported.
Outcomes for Modestly Hypofractionated and Conventionally Fractionated Regimens
In addition to the studies above, more modestly hypofractionated regimens using proton therapy have been reported for non-small cell lung cancer (Table 10.2). While strictly speaking these fall outside the realm of radiosurgical ablation, they are nonetheless instructive with regard to the high doses that can be achieved with the depth–dose characteristics of particle radiotherapy: Targeting the subset of patients with centrally or superiorly located Stage IA–II non-small cell lung cancers, Chang et al. conducted a Phase I/II prospective study of proton radiation with a total dose of 87.5 CGE at 2.5 CGE per fraction [41]. At initial reporting of outcomes with a median follow-up time of 16.3 months, the most common adverse effects were Grade 2 dermatitis (67 %), Grade 2 fatigue (44 %), Grade 2 pneumonitis (11 %), Grade 2 esophagitis (6 %), and Grade 2 chest wall pain (6 %). No grade 4 or 5 toxicities occurred, and the local control rate was 88.9 %. Similarly, Shioyama and colleagues reported 28 patients with Stage I NSCLC treated with proton therapy to a median total dose of 76 CGE in median 3 CGE fractions (range, 2–6 CGE) [42]. The 5-year local control rate was 89 % for patients with Stage IA disease with one case of Grade 3 acute toxicity observed.
Table 10.2
Modestly hypofractionated proton radiotherapy for non-small cell lung cancer
Study | Dose (CGE)/no. of fractions | Chemotherapy | Local control | Overall survival |
|---|---|---|---|---|
Early stage | ||||
Chang et al. [41] (2010) | 87.5/35 | N/A | 89 % (2 years) | 54.5 % (2 years) |
Shioyama et al. [42] (2003) | 76a/22a | N/A | T1A—89 % (5 years) | T1A—70 % (5 years) |
T1B—39 % (5 years) | T1B—16 % (5 years) | |||
Locally advanced | ||||
Chang et al. [45] (2011) | 74/37 | Carboplatin/paclitaxel | 80 % (1 year) | 86 % (1 year) |
Nakayama et al. [43] (2011) | 67.1–91.3/33 | None | 66 % (2 years) | 59 % (2 years) |
Interestingly, the outcomes achieved in the Chang and Shioyama studies with modest hypofractionation were comparable to photon-based ablative approaches. This suggests that, in certain settings, the radiobiological advantages of high LET radiation can substitute for the radiobiological advantages of hypofractionation, while still allowing for the interfractional recovery of normal tissues. In cases where normal tissue toxicity is not a major concern, it stands to reason that combining ablative hypofractionation with charged particle radiotherapy would result in maximal efficacy.
Finally, in the locally advanced setting, several studies have confirmed the utility of using charged particles, specifically protons, to increase the radiation dose while sparing normal tissue (Table 10.2). Though these results also do not reflect a stereotactic approach, they are nonetheless instructive for demonstrating the advantages of proton therapy for treating large volumes in the thorax. Especially notable are the low rates of severe toxicity observed despite the use of high radiation doses and/or concurrent chemotherapy in this setting. Nakayama et al. retrospectively reviewed 35 patients with stage II or III NSCLC who were medically inoperable or who refused surgery and instead were treated with proton therapy without concurrent chemotherapy [43]. The median proton dose delivered was 78.3 CGE (range, 67.1–91.3 CGE). The overall and local progression-free survival rates were 81.8 % and 93.3 %, respectively, at 1 year and 58.9 % and 65.9 %, respectively, at 2 years, and incredibly, no grade 3 or higher toxicity was observed. Similarly, Sejpal et al. published a retrospective analysis of 62 patients with locally advanced NSCLC who were treated with proton therapy and concurrent platinum- or taxane-based chemotherapy and compared their outcomes with those of patients treated in earlier eras with photons [44]. The median total radiation dose was 74 CGE for the proton group and 63 Gy for the photon group. The proton patients had lower rates of pneumonitis, esophagitis, and hematologic toxicity than those observed in patients in the photon cohort despite the fact that the proton dose was, on average, higher. Finally, Chang et al. completed a phase II study of 44 patients with stage III NSCLC who received 74 CGE via conventional fractionation (2 CGE per fraction) with weekly concurrent carboplatin and paclitaxel [45]. Despite the very high intensity of this treatment course, grade 3 toxicities were minimal: 5 had grade 3 esophagitis, 5 had grade 3 dermititis, and only 1 patient had grade 3 pneumonitis. No grade 4 or 5 toxicities were observed. Eighty percent local control and 29 months overall survival were achieved. The concept about combining stereotactic radiotherapy and hypofractionated radiotherapy using intensity-modulated proton-based integrated boost and/or dose painting has been proposed to further improve the therapeutic ratio and improve cure rate in stage III NSCLC.
Summary
While the studies above demonstrate promising tumor control after the use of charged particles in the setting of lung cancer, an important caveat is that these experiences are characterized by considerable heterogeneity with respect to doses, fractionation schemes, and techniques for positioning, immobilization, respiratory compensation, and geometric verification. Nonetheless, they uniformly demonstrate proof of principle that charged particles achieve high local control while limiting normal tissue effects in the thorax. Whether the costs of charged particle radiation are justified by these advantages, however, is controversial until a prospective randomized study can be completed. Fortunately, a trial at MD Anderson Cancer Center is underway to compare stereotactic proton and photon treatments in centrally located stage I, selective stage II, and recurrent NSCLC using a dose regime of 50 Gy in four fractions. The results of this study will shed light on whether the financial cost of proton therapy, specifically, is justified by improved clinical outcomes.
Hepatocellular Carcinoma
Rationale for Particle Therapy for Hepatic Tumors
The liver is a parallel organ and, as such, is particularly sensitive to the toxic effects as more functional subunits are irradiated. Therefore, photons’ integral dose distribution presents challenges when high doses are desired for a target within the liver. In point of fact, hepatocellular carcinoma often arises in the background of chronic hepatic insufficiency, making the dosimetric characteristics of charged particles even more appealing for sparing still-functioning hepatocytes. Additionally specific patient populations may garner additional benefit. For patients with liver transplants, the ability to reduce integral dose with charged particles may have important ramifications for reducing secondary malignancy in a population characterized by an immunocompromised state [46]. For those with portal venous thrombosis a large volume of liver often requires treatment and, often, the size and location of the carcinoma and thrombosis preclude conventional photon radiotherapy at higher doses [47, 48].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree