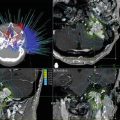
Fig. 47.1
Treatment algorithm for intracranial AVMs
As an alternative to surgical resection, SRS has been shown to be a safe and effective method to manage patients with cerebral AVMs. In 1972, Dr. Ladislau Steiner and colleagues from the Karolinska Institute recognized that single fraction, high-dose irradiation caused the progressive obliteration of AVMs and subsequent cure from the risk of later hemorrhage [30]. In fact, because AVMs could be visualized through angiography before the development of axial imaging, AVMs were a common indication treated in the early radiosurgical series of Leksell. From 1968 until 1982, 204 of the first 762 patients (27 %) having Gamma Knife radiosurgery had AVMs [31]. Concurrent with the work of Leksell and Steiner, Kjellberg and Fabrikant were using heavy charged particles instead of photons to irradiate AVMs [32, 33], and other centers soon developed modified linear accelerators (LINAC) to perform AVM radiosurgery [34–36]. Similar to the Swedish experience, these innovators noted that focused radiation techniques could obliterate a high percentage of irradiated AVMs.
Observation is also a recognized management strategy for AVM patients especially if the AVM has not previously bled. The decision to treat an AVM is simply a comparison of the estimated risk of intervention to the calculated risk if the malformation is left untreated. If the risk of treatment is perceived to be less than the lifetime risk based on the natural history of untreated AVMs, then treatment is recommended. Prior to advent of modern imaging, improved microsurgical techniques, and SRS, the majority of AVMs were not treated directly, and patients discovered to have AVMs were treated for their seizure disorder or headaches. Yet, once it was recognized that selected AVM patients could undergo treatment directed at the AVM with acceptable morbidity, the trend shifted so that surgical excision or radiosurgery was recommended for the majority of patients [37]. Despite technological advances, it is recognized that the risk of treatment for patients with large AVMs is not insignificant, and observation of Spetzler–Martin Grade IV and V AVMs is generally recommended unless the patient has hemorrhaged or is suffering from a progressive neurologic deficit [38].
Recently a number of factors have led some investigators to reexamine observation for patients discovered to have unruptured brain AVMs [39]. First, the annual rate of bleeding for patients with unruptured brain AVMs may be as low as 1 % [40, 41]. Second, the morbidity associated with AVM bleeding may be less than previously thought [42, 43]. A review of 119 AVM patients found that 47 % of patients suffered no disability related to their first ICH and an additional 37 % of patients remained independent in their activities of daily living [42]. Third, neurologic deficits after surgical resection of AVMs may be greater than previously described. Hartmann et al. reported outcomes for 124 AVM patients followed prospectively after surgical resection [44]. At last follow-up, 38 % of patients had new postoperative neurologic deficits. Six percent of patients were disabled after surgery. Factors related to a decline in neurologic function were female gender, AVM size, and deep venous drainage. Likewise, Lawton et al. found that patients with unruptured AVMs were 2.3 times more likely to experience a decline in their modified Rankin Score (MRS) compared to patients with ruptured AVMs [45]. A randomized trial of unruptured brain arteriovenous malformations (ARUBA) was designed to compare the risk of observation versus prophylactic intervention for patients diagnosed with unruptured BAVM [46]. The primary endpoints of the ARUBA trial are the composite risk of death or stroke and risk of death or clinical impairment, defined as a MRS ≥ 2. Although the design of ARUBA has been criticized for a number of reasons including the intended follow-up period after randomization (planned, 5–10 years) [47], it was funded by the National Institute of Neurological Disorders (U01 NS051483) and began enrolling patients in April 2007 at cerebrovascular centers around the world.
AVM Radiosurgery
Patient Selection
Proper patient selection is essential for successful AVM radiosurgery. Once it has been determined that intervention is preferred over observation, several factors must be considered when considering radiosurgery for AVM patients. Most importantly, has the patient bled, and if so, how recently? Patients with a recent ICH and a surgically accessible AVM typically are best managed with surgical resection. However, patients with a recent hemorrhage and a surgically inaccessible AVM are generally good candidates for radiosurgery assuming that the AVM is not too large. Also, patients with a hemorrhage months or years earlier can certainly be considered for radiosurgery because they have passed the time when a re-hemorrhage is most likely to occur. In this situation, a comparison of the chance of AVM elimination without new deficits risk between surgical resection and radiosurgery should be undertaken. Standardized scales such as the Spetzler–Martin Grade [23] and the radiosurgery-based arteriovenous malformation score (RBAS) [48, 49] should be used to estimate the efficacy of surgical resection and radiosurgery, respectively, for individual AVM patients. Of course, the success of any intervention must take into account the physician’s experience with a particular group of patients, rather relying on published reports from experienced centers.
Other considerations regarding treatment choice include the presence or absence of associated aneurysms, history of seizures, and need for pretreatment embolization. Aneurysms on feeding vessels of AVMs are likely caused by the stress on the arterial walls due to increased blood flow to the AVM. Patients with associated aneurysms and surgically accessible AVMs can often have both operated in a single procedure, thereby immediately removing the hemorrhage risk. Patients with associated aneurysms and surgically inaccessible AVMs can be considered for pre-radiosurgical surgical clipping or endovascular treatment of their aneurysm, with radiosurgery performed during a separate later procedure. Conversely, if the associated aneurysm is small, radiosurgery alone is generally sufficient because the aneurysm will likely either stabilize or regress as the blood flow to the AVM decreases after radiosurgery.
Another consideration when discussing surgical resection or radiosurgery for AVM patients is a history of seizures. Approximately 15–20 % of AVM patients will present with a seizure [50, 51]. However, few patients will have medically resistant epilepsy, and the use of standardized scales to determine seizure frequency is uncommon in AVM papers. Piepgras et al. studied seizure outcomes after AVM surgery [51]. Preoperative seizure burden was defined as less or more than four seizures prior to treatment. In the “low-seizure group” with a follow-up longer than 2 years, 93 % were seizure-free, 2 % improved but continued to have seizures, and 5 % had worsening of their seizures. In patients with more than four seizures preoperatively, seizure freedom measured at 2 years of follow-up or longer was achieved in 76 % of patients, 21 % were improved, and 3 % remained unchanged. Overall, 83 % of patients remained seizure-free at last follow-up in this study. Likewise, Han et al. reviewed 440 patients undergoing resection of supratentorial AVM [50]. Preoperative seizures were associated with prior AVM hemorrhage, male sex, and frontotemporal lesion location. At a mean follow-up of 21 months, 96 % of patients had a modified Engel class I outcome (seizure-free, 80 %; one postoperative seizure, 16 %). We retrospectively reviewed 65 AVM patients with one or more seizures having SRS at our center between 1990 and 1998 [52]. Twenty-six patients (51 %) were seizure-free (aura-free) after radiosurgery at 3-year follow-up; 40 patients (78 %) had an excellent outcome (non-disabling simple partial seizures only) at 3-year follow-up. Factors associated with seizure-free or excellent outcomes include a low seizure frequency score (<4) before radiosurgery, smaller AVM volume, and smaller AVM diameter. Twenty-three patients had intractable partial epilepsy prior to treatment. Twelve (52 %) of 23 and 11 of 18 (61 %) patients with medically intractable partial epilepsy had excellent outcomes at years 1 and 3, respectively. Yang et al. analyzed 161 consecutive patients having SRS for unruptured AVM (mean follow-up, 90 months) [53]. Sixty-six of 86 patients (77 %) were seizure-free after SRS. In this series, AVM obliteration correlated with a seizure-free outcome after SRS (97 % versus 31 %). Although the number of patients who are seizure-free appears higher in most studies after AVM resection, selection bias in these anecdotal series prevents any conclusion as to which approach correlates with improved seizure outcomes.
Technique
The goal of SRS is to accurately deliver a high dose of radiation to an imaging-defined target. Although the definition of SRS has been modified to include treatment in 1–5 fractions, the majority of patients with intracranial AVMs treated with SRS are treated in a single fraction. Thus, placement of a stereotactic headframe is generally performed to ensure rigid fixation and minimize patient movement during imaging and radiation delivery. Headframe placement for adults is performed under local anesthesia supplemented by a low dose of benzodiazepine. Patients less than 16 years typically require general anesthesia for radiosurgery. Once the stereotactic headframe has been placed, most patients undergo a post-gadolinium MRI and biplanar cerebral angiography. Reliance on angiography alone for radiosurgical dose planning increases the chance of treating too much adjacent normal brain tissue due to the often irregular shape of AVMs [54]. In addition, for AVMs located in the posterior fossa and lateral temporal regions, the chance of not including a portion of the nidus in the prescription isodose volume (PIV) is greater secondary to the difficulty in visualizing the AVM clearly on angiography. Over the past 15 years, we have become increasingly confident in performing AVM SRS without a stereotactic angiogram and now perform approximately 20 % of our cases based on MRI alone. Patients with small, compact, hemispheric AVMs with simple venous drainage are ideal candidates for using MRI alone for dose planning. Nonetheless, we continue to insist that patients have a complete diagnostic angiogram, including appropriate external carotid injections, before any decision is made about the feasibility of SRS and to determine if the patient has any associated aneurysms.
The goal of SRS planning is to create a conformal dose plan that precisely covers the three-dimensional shape of the nidus. Feeding arteries and draining veins are not included in the dose plan if possible. Inclusion of these vessels will increase the volume covered, which may decrease the radiation dose prescribed. Dose prescription must take into account two conflicting considerations: the chance of obliteration versus the chance of radiation-related complications. Increasing radiation dose directly correlates with the chance of obliteration. Assuming that the radiation is well targeted, the chance of AVM cure is approximately 70 %, 80 %, and 90 % for radiation doses of 16, 18, and 20 Gy, respectively [55, 56]. However, the likelihood of radiation-related complications after AVM radiosurgery increases at higher radiation doses and larger AVM volumes. Early dose prescription generally followed either Kjellberg’s 3 % isodose line [32] or Flickinger’s integrated logistic formula [57] to predict the probability of radiation-related complications. More recent studies have correlated the chance of radiation-related complications after AVM radiosurgery relates to some measure of the radiation dose to the surrounding tissue [58, 59]. Patients with AVMs in the thalamus, basal ganglia, and brainstem are more likely to develop neurologic deficits secondary to imaging changes noted on MRI [58].
After the dose plan is reviewed by all members of the radiosurgical team and found to be acceptable, the patient undergoes radiation delivery. Following radiation delivery, the patient is typically discharged home the day of the procedure. Immediate complications are rare. Some patients complain of pin site discomfort, neck pain, or headache, but these are usually temporary and can be managed with over-the-counter medications. Follow-up after radiosurgery typically consists of clinical examination and MRI at yearly intervals for the first 5 years after SRS. If MRI suggests that the AVM has gone on to complete obliteration, then follow-up angiography is recommended to confirm cure [60, 61]. Patients with residual AVM on follow-up angiography are evaluated for observation, repeat SRS, or surgical resection based on their age, clinical condition, and the AVM response from the first radiosurgical procedure.
Obliteration After Radiosurgery
The primary goal of AVM radiosurgery is complete nidus obliteration to eliminate a patient’s risk of future hemorrhage. Lindquist and Steiner in 1988 defined AVM obliteration as follows: “we have considered a result satisfactory only when the arteriogram has shown a normal circulation time, complete absence of pathological vessels in the former nidus of the malformation, and the disappearance or normalization of draining veins from the area” [62]. Generally, AVM obliteration occurs between 1 and 5 years after radiosurgery. Sequential histopathological changes after AVM radiosurgery include early damage to the endothelial cells, followed by progressive thickening of the intimal layer secondary to proliferation of smooth muscle cells which produce an extracellular matrix containing Type IV collagen, then cellular degeneration and hyaline transformation [63]. The effect of high-dose single fraction radiation has been studied in cell cultures taken from patients undergoing AVM resection [64]. Within 5 days after irradiation, the proliferation index decreased and remained decreased over the observation period. Other notable findings were immunohistochemical evidence of apoptosis and transformation of fibroblastic cells into activated myofibroblasts. Histologic and electron microscopic studies of seven AVMs resected after bleeding 10–52 months after radiosurgery revealed spindle cell proliferation in the connective tissue stroma and in the subendothelial region of irradiated vessels [65]. It was concluded that the characteristics of the spindle cells were similar to myofibroblasts noted during wound healing, and these cells likely contributed to the occlusive process and final obliteration of AVMs after radiosurgery.
The most important factor associated with obliteration after AVM radiosurgery is the margin dose delivered to the nidus [55, 56, 66–69]. A number of models have been developed to predict the chance of AVM cure after SRS. Karlsson et al. reported the K index as a method to predict obliteration after AVM radiosurgery [56]. Based on 945 AVM patients having radiosurgery from 1970 until 1990, they found a logarithmic relationship between minimum dose and AVM obliteration increasing to a maximum of 87 %. A higher average dose also shortened the latency to AVM obliteration. The correlation of obliteration rate to the product minimum dose (AVM volume)1/3 was termed the K index. The obliteration rate increased linearly with the K index up to a value of approximately 27, and for higher K values, the obliteration rate had a constant value of approximately 80 %. For the group of patients receiving an AVM margin dose of at least 25 Gy (n = 273), the obliteration rate at 2 years was 80 %. If obliterations that occurred beyond 2 years are included, the obliteration rate increased to 85 %. Schwartz et al. developed the obliteration prediction index (OPI) as a method to predict success or failure for individual AVM patients [70]. Analyzing a total of 426 patients having either Gamma Knife or LINAC-based SRS, a relationship was noted between the calculated OPI (AVM margin dose, Gy/lesion diameter, cm) and AVM obliteration. Although both the K index and the OPI correlate with AVM elimination, neither takes into account the likelihood of producing radiation-related complications for a given radiation dose.
Perhaps more instructive than studies analyzing factors relating to AVM obliteration has been a number of papers that have reviewed patients with incompletely obliterated AVMs after SRS [71–73]. The most common reasons for incomplete nidus obliteration are targeting errors, recanalization of a portion of the AVM that was previously embolized, reexpansion of nidus after hemorrhage, and low radiation dose. These studies have demonstrated the need for complete nidus coverage at the time of SRS. The routine use of CT or MRI in conjunction with angiography has been the most important change to reduce the chance of “missing” a portion of the AVM with the PIV [54]. It is also critical that all possible feeding arteries are injected at the time of SRS, including the external carotid arteries for peripherally located AVMs. Certainly part of the problem in AVM SRS is defining the nidus accurately. Buis et al. had six independent clinicians contour the nidus of AVM patients based off digital subtraction angiography (DSA) [74]. They noted significant interobserver variation when outlining the nidus and concluded that this may contribute to failure in some AVM radiosurgical cases. Yu et al. compared dose plans based on a combination of angiography and MRI to those derived from MRI alone [75]. They concluded that MRI-based dose planning without angiography should be limited to patients with smaller AVMs and compact niduses. A study from the University of Florida has suggested that AVM morphology is an important factor associated with obliteration [76]. Specifically, they noted patients with a diffuse nidus structure and associated neovascularity were at a higher risk of incomplete nidus obliteration when compared to patients with compact AVMs. High-flow AVMs have also been noted to have a lower rate of obliteration compared to low-flow lesions. In a study of 139 patients, patients with angioarchitectural features consistent with high-flow achieved had a complete obliteration rate less than 40 % [77].
Post-radiosurgical Hemorrhage
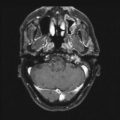
The primary drawback of AVM SRS when compared to surgical resection is that the patient remains at risk for hemorrhage until the AVM has gone onto complete obliteration (Fig. 47.2). The issue of AVM bleeding during this latency interval has been extensively studied. Early papers on AVM radiosurgery suggested that the risk of bleeding initially increased before AVM obliteration occurred [35, 78]. Colombo et al. reported 180 patients having LINAC-based AVM radiosurgery [35]. Twenty-seven patients with large AVMs underwent partial treatment; the mean follow-up in this series was 43 months. Fifteen patients bled after radiosurgery. In totally irradiated cases, the bleeding risk decreased from 4.8 % in the first 6 months after SRS to 0 % starting from 1 year after SRS. Patients with partially irradiated AVMs had bleeding 4–10 % over the first 2 years, then no bleeding thereafter. A review of 65 patients with Spetzler–Martin Grade I–II AVMs found that five patients (8 %) sustained an ICH after radiosurgery [78]. Although the annual hemorrhage rate was 3.7 % over the latency interval, all the observed hemorrhages occurred within the first 8 months after the procedure. Despite these early papers suggesting an increased risk of bleeding after radiosurgery, larger and more detailed analysis of this question has confirmed that the risk of bleeding is either unchanged or decreased following AVM SRS [79–83]. Maruyama et al. performed a retrospective observational study of 500 AVM patients having radiosurgery [83]. Comparing the risk of bleeding before and after radiosurgery, they found a 54 % reduction in the bleeding risk during the latency interval. The risk reduction was greatest among patients who presented with a hemorrhage. Likewise, Karlsson et al. analyzed the large AVM experience at the Karolinska Institute and found that some measure of protection occurred as early as 6 months after radiosurgery for patients receiving an AVM margin dose of 25 Gy [82]. The presence of nidal or associated aneurysms has also been associated with an increased risk of ICH after AVM radiosurgery [80, 81]. Following angiographic obliteration, the chance of hemorrhage is markedly reduced, but cases of ICH after nidus elimination have been reported [84, 85]. Risk factors for postobliteration bleeding include young age and persistent enhancement of the irradiated nidus.


Fig. 47.2
Neuroimaging studies of a 26-year-old woman with an incidentally discovered left basal ganglia AVM. Lateral (Left, a) and anterior-posterior (Middle, b) left internal carotid angiograms showing AVM on the day of SRS. (Right, c) Computed tomography performed 6 months after SRS showing both intraparenchymal and intraventricular hemorrhage
Radiation-Related Complications
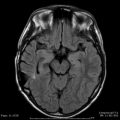
Advances in imaging and radiosurgical devices have significantly improved patient outcomes after SRS. Nonetheless, the tissue adjacent to a radiosurgical target does receive a dose of radiation. Assuming that the dose plan conforms to the three-dimensional shape of the target, the major factors that determine the amount of radiation delivered to nearby structures are the PIV and the prescribed radiation dose. As the PIV increases, the fall off of radiation becomes less steep, resulting in more radiation to the adjacent brain. It is this general principle that typically limits the size of intracranial radiosurgical targets to 3 cm or less in average diameter. Recognition of the relationship between the overall radiation exposure and the chance of radiation-related complications has resulted in studies correlating imaging changes on MRI to measures of radiation such as the 12 Gy volume (V12) [58] and the mean dose received by 20 cm3 surrounding the maximum radiation point (Dmean20) [59]. Areas of increased signal on long-TR MRI are noted in approximately 30–50 % of AVM patients after SRS. The chance of developing these imaging changes increases at larger V12 or Dmean20. Levegrun et al. studied the correlation of radiation-induced imaging changes and dose distribution parameters in 73 patients having AVM radiosurgery [86]. The AVM target volume correlated significantly with the development of edema and breakdown of the blood–brain barrier. They concluded that each measure studied (including V12 and Dmean20) yielded similar results, and no parameter was favored over the others. Yet, although such imaging changes are frequently noted within the first year after AVM radiosurgery, most are temporary and the majority of patients remain asymptomatic (Fig. 47.3). It is believed that many “radiation-related” imaging changes noted after AVM SRS are related to alterations in the regional blood flow in the brain adjacent to the AVM as the nidus obliterates and blood is being directed into vasculature that has lost the capability for autoregulation. Early closure of draining veins before occlusion of the nidus may also be a factor that contributes to the development of edema after SRS (Fig. 47.4) [87, 88]. Patients with AVMs in the thalamus, basal ganglia, and brainstem are more likely to develop neurologic deficits secondary to imaging changes noted on MRI [58, 67, 68, 89].



Fig. 47.3
Long-TR MRI of a 10-year-old boy who presented with headaches. (Left, a) MRI before SRS showing the right-sided AVM involving the sensorimotor cortex. (Middle, b) MRI 9 months after showing the nidus to be smaller and adjacent edema. The patient continued to have headaches, but was neurologically intact. (Right, c) MRI 26 months after SRS showing near-complete resolution of the edema

Fig. 47.4
MRI of a 49-year-old woman with an incidentally discovered left frontal AVM. (Left, a) Long-TR image before SRS. Eighteen months after SRS the patient suffered a generalized seizure. Long-TR (Middle, b) and T1-weighted without gadolinium (Right, c) MRI at that time showed the nidus to be decreased in size with adjacent edema. Note acute thrombus within nidus and the draining veins
Although most imaging changes detected on MRI after AVM SRS are temporary and resolve by 2 years, a small percentage of patients will continue to demonstrate imaging changes consistent with radiation necrosis (Fig. 47.5). MRI findings consistent with radiation necrosis include persistent enhancement at the irradiated site beyond 2 years with associated edema and mass effect. Again, the chance that a patient will have symptoms related to radiation necrosis relates primarily to the location of the AVM. In addition to radiation necrosis, other late complications have been noted after AVM radiosurgery including diffuse white matter changes [90], cyst formation [91–93] (Fig. 47.6), and stenosis of major intracranial vessels [94]. The most devastating complication after any radiation-based procedure is a radiation-induced neoplasm. To date, only a few cases of radiation-induced tumors have been reported after AVM radiosurgery [95]. Although the actual incidence of this complication will not be known for years, it is estimated that the risk of a radiation-induced tumor after radiosurgery will be significantly less than fractionated radiation therapy [96].



Fig. 47.5
Post-gadolinium (left) and long-TR (right) MRI performed 3 years after SRS of a left sylvian fissure AVM demonstrating persistent enhancement and edema consistent with radiation necrosis

Fig. 47.6
MRI of a 35-year-old woman who underwent LINAC-based SRS 7 years earlier for an incidentally discovered left frontal AVM. The patient developed increasing headaches, seizures, and syncope. (Top, left, a, and right, b) Post-gadolinium and long-TR images showing cyst formation, edema, and mass effect. Angiography revealed complete obliteration. (Bottom, left, a, and right, b) Post-gadolinium and long-TR images 2 months after surgical resection of the obliterated AVM showing resolution of edema and mass effect
Repeat AVM Radiosurgery
Patients with residual nidus after AVM radiosurgery remain at risk for ICH. A number of studies have analyzed the results of repeat AVM radiosurgery [72, 97–99]. Karlsson et al. reviewed 112 patients and tested whether models developed after one radiosurgery also predicted obliteration and complication rates after repeat AVM radiosurgery [97]. Sixty-two of 101 patients with angiographic follow-up had complete obliteration (predicted number of obliterations, 65). Fourteen patients (13 %) had radiation-related complications after a second radiosurgical procedure. They concluded that the obliteration rate after repeat radiosurgery is similar to primary procedures, but the complication rate increases with the overall amount of radiation given. Two recent studies from the University of Florida [99] and the University of Pittsburgh [72] confirmed that complete obliteration is achieved in approximately 70 % of patients after repeat SRS. Patients with smaller volume AVMs at the time of repeat SRS had higher rates of obliteration in both of these series. Kano et al. found that the incidence of radiation-related complications increased from 5 % after initial SRS to 10 % after repeat SRS [72], whereas Stahl et al. noted no change in the risk of radiation-related complications between the first and second procedures [99]. Overall, repeat SRS should be considered a safe and effective option for patients with incomplete AVM obliteration after their initial radiosurgical procedure.
Radiosurgery of Large AVMs
Although AVM obliteration is significantly related to higher radiation doses, dose prescription must also take into account the likelihood of radiation-related complications. Studies on the dose–volume relationship of AVM post-radiosurgical radiation-related complications have demonstrated a high morbidity for large AVMs [58, 59, 86]. Miyawaki et al. reported that following linear accelerator-based radiosurgery of AVMs greater than 14 cm3 receiving 16 Gy or more, the incidence of post-radiosurgical abnormalities on long-TR MRI was 72 % [100]. The incidence of radiation necrosis for these patients was 22 %. Consequently, single-fraction SRS is generally performed only for patients with AVMs of an average diameter of 3 cm or less (approximately 14 cm3).
Alternative strategies that utilize radiation in the management of large AVMs include embolization followed by SRS, fractionated radiation techniques, and staged-volume SRS. Planned embolization and SRS has been used for many years to manage patients with large AVMs as an alternative to surgical resection for these difficult lesions [101–104]. As opposed to embolization prior to resection where flow reduction is the goal, the goal of pre-radiosurgical embolization is permanent volume reduction. Gobin et al. published the results in 96 patients undergoing acrylate embolization followed by radiosurgery [102]. Complete AVM obliteration was documented in 53 of 90 evaluable patients (59 %). Sixteen patients (13 %) had complications from the embolization procedures, and two patients died of intracerebral hemorrhages prior to having radiosurgery. Recanalization of the AVM was seen in 14 % of patients. Blackburn et al. described the management of 21 patients with large AVMs (>3cm) using combined embolization and SRS from 1994 to 2006 [101]. Eight complications occurred in 43 embolization procedures (24 %). The majority of complications were temporary and no patient had a permanent major neurologic deficit. Following SRS, 16 of 19 patients (84 %) had obliteration confirmed by either angiography (n = 13), MR angiography (n = 2), or CT angiography (n = 1). They concluded that endovascular therapy followed by SRS was an effective and safe method to treat large AVMs not amenable to standard surgical or SRS treatment.
Conventional fractionated radiation therapy has been used to treat patients with large AVMs [22, 105]. Redekop et al. reported 15 patients with inoperable AVMs ranging from 1.5 to 6.5 cm in diameter who underwent conventional radiation therapy between 1955 and 1985 [105]. Fourteen of 15 patients received a total radiation dose of 40 Gy or more in 15–28 fractions. At a mean follow-up of 8.1 years, the annual hemorrhage rate was 3.3 %, and later angiography confirmed AVM obliteration in 2 of 12 patients (17 %). Karlsson et al. reviewed the outcomes of 28 AVM patients undergoing fractionated radiation therapy between 1980 and 1985 [22]. The median volume treated was 78 cm3. Using a fractionation scheme of 42 Gy in 12 fractions, only two patients (8 %) demonstrated angiographic cure. The annual hemorrhage rate after radiation therapy was 6 %. Although the information on low dose fractionation for AVM patients is limited, it appears that little protection is provided against future bleeding after this technique.
More recently, a number of centers have examined the efficacy of hypofractionated radiation schedules using higher radiation doses per fraction to treat patients with intracranial AVMs [106–109]. Lindvall et al. used several fractionation schemes (generally 30–35 Gy/five fractions) to treat 36 AVMs patients [108]. Two-year follow-up angiography showed that 48 % of the patients were cured; angiographic obliteration rose to 76 % on 5-year angiography. Although the median AVM volume was only 8.5 cm3 for the entire group, obliteration for patients with AVMs larger than 10 cm3 (n = 10) was 70 % at last follow-up. Veznedaroglu and colleagues from Jefferson Hospital managed 30 patients with large AVMs from 1995 to 1998 using a combination of pre-radiation embolization and stereotactic radiation therapy (SRT) [109]. Patients received either 42 Gy in six fractions (n = 7) or 30 Gy in six fractions (n = 23). Angiographic obliteration was noted on 5-year follow-up angiography in 83 % of patients in the 42 Gy group (five of six patients) compared to only 22 % of patients in the 30 Gy group (4 of 18 patients). However, the overall morbidity in the higher dose group was 43 % (14 % permanent deficits). Chang et al. treated 33 AVM patients with SRT using a treatment regimen of 25–35 Gy in four daily fractions [106]. Fifteen of the 33 patients (45 %) had AVMs larger than 2.5 cm in diameter. The 5- and 6-year actuarial obliteration rates were 61 % and 71 %, respectively. One patient (3 %) developed radiation necrosis after SRT. Hattangadi et al. reported 59 patients having planned two-fraction stereotactic proton beam SRS from 1991 to 2009 [107]. The median AVM volume was 23 cm3; the most common dose prescription was 16 Gy radiobiologic equivalent in two fractions. At a median follow-up of 56 months, nine patients (15 %) had total obliteration. Five patients (9 %) had permanent complications after proton-beam therapy, none of which were severe.
Recognition of the limitations of the various management options available to patients with large AVMs has encouraged some radiosurgical centers to begin staged-volume radiosurgery for these patients [110–112]. Volume staging of large AVMs into multiple radiosurgical sessions separated by several months allows a higher radiation dose to be delivered to the entire AVM volume while minimizing the radiation exposure to the adjacent brain. We performed a dosimetric study comparing staged-volume AVM radiosurgery and hypothetical single-session procedures for our first ten patients [112]. Staged-volume radiosurgery decreased the V12 by an average of 11.1 %, and the non-AVM V12 was reduced by an average of 27.2 %. Huang et al. reported the outcomes for 18 patients with large AVMs (>15cm3) undergoing staged-volume SRS over a 13-year interval [110]. The median radiation dose at each stage was 15 Gy. The 5-year rate of complete obliteration and hemorrhage after SRS was 29 % and 31 %, respectively. One patient (6 %) had a radiation-related neurologic deficit. Kano et al. treated 47 patients with staged-volume SRS between 1992 and 2006 [111]. The median total volume was 22.0 cm3; the median AVM margin dose was 16 Gy. The 5-year actuarial obliteration rate was 28 %. Patients treated with doses ≥ 17 Gy had a 5-year obliteration rate of 62 %. Radiation-related complications occurred in 13 % of patients. By minimizing the radiation exposure to the adjacent brain, the incidence of radiation-related complications has been reduced after staged-volume SRS for large AVMs compared to single-fraction SRS [100]. More data are need on this relatively new technique to permit a better comparison with other available treatment options for this difficult patient group.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree