First Author (year)
Methods compared
Patients (N)
PTV (cm3)
Results
Bourlandet al. (‘94) [9]
Noncoplanar circular arc vs. conformal fixed beam
NA
NA
Smaller high-dose volume and better homogeneity for fixed beam
Hamilton et al. (‘95) [99]
Noncoplanar circular arc vs. conformal fixed beam
1
3.5
Smaller high-dose volume and better homogeneity for fixed beam
Shiu et al. (‘97) [100]
Noncoplanar circular arc vs. conformal fixed beam
2
4.5–9
Smaller high-dose volume and better homogeneity for fixed beam
Kubo et al. (‘97) [101]
Noncoplanar circular arc vs. conformal fixed beam
11
0.4–17.6
Lower doses to normal tissues and shorter planning time for fixed beam
Kramer et al. (‘98) [102]
Noncoplanar circular arc vs. IMSRT
1
NA
Better CI, lower max. Dose, better HI, but larger penumbra in IMSRT
Yu et al. (‘99) [14]
Noncoplanar circular arc vs. IMSRT vs. conformal fixed beam
3
9.6–36.7
IMSRT: better CI, lower dose to normal brain than others
Cardinale et al. (‘98) [103]
Noncoplanar circular arc vs. conformal fixed beam vs. IMSRT
3
11.5
Depending upon shape: IMSRT better conformity and OAR-sparing (irregular) or arcs better (ellipsoid)
Benedict et al. (‘01) [104]
Noncoplanar circular arc vs. conformal fixed beam-IMSRT
4
2.3–3.5
IMSRS: lower dose to OAR, lower volume normal brain receiving >50 % of prescr. Dose
Leavitt et al. (‘01) [105]
DCA vs. IMSRS/IMSRT
3
8.7–55
Lower dose to OAR in IMSRS/IMSRT
Yu et al. (‘02) [10]
3D conformal fixed beam vs. DCA vs. IMSRT
50
Large volumes, no SRS
DCA: best conformity, lower dose to normal tissues than conformal beam but not IMSRT
Perks et al. (‘03) [13]
Gamma Knife vs. Conformal Fixed Beam vs. DCA
8
0.3–10.6
Gamma Knife: best conformity, fixed beam and arc : better homogeneity
Baumert et al. (‘03) [11]
DCA vs. IMSRS
10
15–43
IMSRS: better coverage and lower OAR dose, but higher low-dose regions in the normal brain
Ernst-Stecken et al. (‘05) [106]
DCA vs. IMSRS
6
1.1–10.4
IMSRS: lower volume of normal brain receiving >90 % of PD but higher integral dose. RTOG criteria best met by DCA
Dose Limitations at the Skull Base
Radiosurgery in the region of the skull base poses special challenges, as many critical structures converge there, most notably the optic apparatus and cranial nerves. Table 38.2 summarizes the current data from literature, which can assist in determining the feasibility of radiosurgery itself or a given plan. We cannot emphasize enough that these data are to be used with utmost caution as, with exception of the RTOG 90–05 data, they are not the result of dose escalation or randomized trials but stem originate mostly from retrospective clinical outcome data, with not only limited long-term outcome data but also small patient collectives.
Table 38.2
Summary of radiosurgical tolerance doses at the base of skull
Organ | # fx | Vol. cm3 | Vol. % | Vol. limit (Gy) | Max. limit (Gy) | |
|---|---|---|---|---|---|---|
Major vessels | 1 | 0.035–10 | 31–37 | 37 | ||
3 | 5–10 | 21–39 | 45 | |||
4 | 1–10 | 35–43 | 49 | |||
5 | 10 | 47 | 53 | |||
Pituitary gland | 1 | NA | ||||
Brainstem | 1 | 1 | 10 | 15 | ||
3 | 1 | 18 | 23 | |||
5 | 1 | 26 | 31 | |||
5 | 100 | 20 | ||||
Brain | 5 | 100 | 20 | |||
Chiasm | 1 | 8–15 | 10 Gy safe → 77 % chance of RON above 15 Gy | |||
3 | 0.2 | 15 | ||||
5 | 0.2 | 100 | 20 | 25 | ||
Cranial nerves | 1 | 20–30 | Fifth cranial nerve 20 Gy max | |||
Cochlea | 1 | 12 | ||||
3 | 20 | |||||
5 | 27.5 | |||||
Lens | 1 | 2–3 | ||||
2 | 3–6 | |||||
3 | 3–7 | |||||
5 | 3–7 | |||||
Retina and lacrimal gland | 1 | 5 | ||||
2 | 5–10 | |||||
3 | 5–15 | |||||
5 | 5–15 | |||||
Neurovascular bundle | 5 | 20–50 | 38 | |||
Optic nerve | 1 | 8–15 | ||||
2 | 10 | |||||
3 | 0.2 | 15 | 19.5 | |||
5 | 0.03–0.5 | 12.5–25 | 25–30 | |||
Area postrema | 6.2 | |||||
From these data, one can surmise that the most critical structures are the optic pathways. The risk of clinically significant radiation optic neuropathy for patients receiving SRS for skull base tumors is 1–2 % following doses to optic chiasm below 10 Gy and this percentage may significantly increase for higher doses [17, 18]. Leber et al. reviewed 50 patients having SRS for benign skull base tumors in which the optic nerves or chiasm were exposed to 4.5 Gy or more. For patients receiving 10–15 Gy and greater than 15 Gy, the risk of radiation-induced optic neuropathy was 26.7 % and 77.8 %, respectively, however no optic neuropathy was observed when a dose less than 10 Gy was delivered to the optic apparatus [19]. Stafford et al. found that the risk of developing a clinically significant optic neuropathy was 1.1 % for patients receiving a maximum point dose of 12 Gy or less [20] and similar results have been reported by others [21]. Considering an effective dose of 13–16 Gy to achieve local control of a given tumor and a recommended dose of 8 Gy as the maximum for the optic chiasm this means that in clinical practice a distance between tumor margin and optic apparatus should be at least of 2–3 mm to avoid visual deterioration.
The auditory apparatus is second in line with 12–15 Gy SRS tolerance using a single fraction or 18 Gy in three fractions. Then follows the trigeminal nerve and finally the motor cranial nerves [2, 4, 6, 7, 9–12] which have rarely been reported having a deficit using doses under 16 Gy.
Considering these data and published risks of optic neuropathy for conventionally fractionated radiotherapy, α/β[alpha/beta] ratios in the range of 0–1 seem reasonable for estimating radiosurgery dose equivalents for optic and cranial nerves.
Re-irradiation at the Skull Base
This often represents a relatively high-risk treatment, as critical tolerance doses have usually been applied in the first course of treatment. Historically, most radiation oncologists have refused re-irradiation due to concern about the risks of late central nervous system toxicity, especially radionecrosis, which may appear several months to years after treatment. Re-irradiation of brain tumors has recently attracted more interest as our understanding of the tolerance of the brain to radiation evolves, and developments in radiation technology and imaging make highly accurate targeting of biologically relevant tumor volumes possible. Prospective data addressing this approach is however lacking.
Obviously, the applicable doses depend to large extent on time since initial treatment, treatment volume, fractionation used and location as well as individual factors such as histology, location, and imminent danger to the patient, so that individual recommendations are well beyond the scope of this book.
However, it seems reasonable to apply the same rationale to re-irradiation in the base of skull regions as is applicable to the brain itself; own and other published clinical experiences have yet to describe any major untoward effects of such re-irradiation if certain radiobiological principles are followed:
Although available data come mainly from animal spinal models, the pathogenesis of radiation toxicity and recovery potential in the brain is assumed to be similar with a similar, low α/β[alpha/beta] [22]. Animal spinal cord models suggest that significant recovery follows irradiation; conservative estimates being up to 50 % recovery within 1–2 years after initial exposure [23, 24]. An increasing body of evidence is available (mainly for re-irradiation of gliomas) resulting in a solid clinical rationale should re-irradiation be required. But there may also be a case to be made for repeat radiosurgery in the base of skull region, i.e. for acoustic neuromas [25].
In summary, re-irradiation at the skull base remains a complex procedure and should be left to centers with extensive experience herein. With developments in molecular-targeted therapy, further exploration of the role of re-irradiation on its own or in combination with novel agents is needed.
Clinical Entities
Skull base tumors are relatively rare. Approximately 0.1 % of all intracranial tumors are chondrosarcomas and also approximately 0.1 % of all intracranial tumors are chordomas. For benign tumors of the skull base such as glomus tumors, local control rates of 90–100 % have been reported. On the other end of the spectrum, local control rates for chordomas range from 50 to 70 %.
On the following pages, the radiosurgical options for primary malignant and benign skull base tumors will be discussed.
Angiofibroma
Disease Pathophysiology
Juvenile angiofibroma is one of the most common benign nasal tumors affecting males between 9 and 19 years of age accounting for 0.05 % of all head and neck tumors [26]. In the USA it is the most common Head and Neck tumor of adolescence [27]. The tumor originates from the broad area of the posterolateral wall of the nasal cavity in the region of the sphenopalatine foramen [28]. The etiology is still unclear, however recent electron microscope studies suggest it is rather a vascular malformation, possibly associated with incomplete regression of the first brachial artery, than a tumor [29]. They often act in a malignant manner by eroding into the surrounding sinuses developing an aggressive growth pattern. Intracranial extension is noted in 10–20 % of cases. Different staging systems based on tumor extension have been proposed [30].
Typical clinical symptoms are frequent epistaxis, nasal obstructions, and rhinorrhea. Chronic rhinosinusitis, swelling of the cheek, alteration of olfaction are possible; unilateral otitis media may result by eustachian tube blockage. By eroding into the cranial fossa, diplopia may occur as well as symptomatic pressure of the chiasm and optic nerves.
Treatment Options
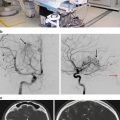
After CT/MRT and bilateral angiography for staging and determination of blood supply, treatment of choice in patients with primary and recurrent juvenile nasopharyngeal angiofibroma is surgical resection as sole treatment of early-stage tumor when gross total resection can be achieved. Preoperative embolization is recommended by most authors to reduce intraoperative blood loss [31]. In the last decade, endoscopic resections have evolved, providing reduction of complications and intraoperative bleeding and thus an alternative to open surgery for early to intermediate stage angiofibromas [30]. Complications in advanced-stage angiofibromas after surgery include intraoperative blood loss requiring transfusions, neuralgia, hearing loss, and ophthalmoplegia [32]. Surgical contraindications include unresectable intracranial involvement.
After primary resection of extracranial angiofibromas, cure rates of nearly 100 % can be achieved compared with results in patients with intracranial lesions where the cure rates are approximately 70 %.
In case of incomplete resection, or in advanced-stage lesions, a combination of surgery followed by radiotherapy is indicated due to the high recurrence rate in these patients. Advanced-stage disease with cranial base involvement and intracranial extension often allows only subtotal resection of the tumor.
External beam irradiation has been shown to be a useful adjunct to therapy in patients with unresectable recurrent disease. Gamma Knife and linac-based radiosurgery with a dose of 20 Gy to the tumor margin (55 % isodose line) is an effective way to deliver high-dose radiation to incompletely resected angiofibromas [32] as they represent slow-growing and late-responding tissues. Therefore, a radiobiological advantage to radiosurgery may be given. Radiosurgery is regarded as a reasonable strategy in small-volume and localized angiofibromas.
For larger angiofibromas, fractionated conformal radiation therapy with total doses of 36–46 Gy is also effective and may reduce the risk of late effects such as cranial nerve deficits, bone and soft tissue necrosis. Another treatment technique described in the literature is the use of intensity-modulated radiation therapy (IMRT) in three cases [33]. The applied tumor dose varied from 34 to 45 Gy. In all three cases, a reduction of tumor size occurred without significant toxicity. Especially in children, inhibition of facial bone growth and second malignancy are severe possible side effects and must be considered in treatment decisions [34–36].
Esthesioneuroblastoma
Disease Pathophysiology
Esthesioneuroblastomas are rare tumors originating from the olfactory epithelium of the upper nasal cavity [36]. The sex distribution of esthesioneuroblastomas is uniform. The olfactory nerves perforate the groove in the ethmoid bone in the cribriform plate and continue into the subarachnoid spaces. Therefore, a high incidence of intracranial extension results. This highly dedifferentiated tumor occurs in all periods of life with a bimodal peak at the second and sixth decades. The two most common clinical signs of esthesioneuroblastoma constitute unilateral nasal obstruction and epistaxis. Other clinical symptoms include headache, swelling of the cheek, blurred vision, and dental pain. Esthesioneuroblastoma can metastasize to regional lymph nodes, primarily of the neck [37] lung, or bones. According to the WHO classification system, the terms olfactory neuroblastoma and olfactory neurogenic tumors are used. The Kadish staging classification is shown in Table 38.3.
Table 38.3
Staging of esthesioneuroblastoma according to Kadish et al.
Stage | Characteristic |
|---|---|
A | Confined to the nasal cavity |
B | Confined to the nasal cavity and one or more paranasal sinuses |
C | Extending beyond the nasal cavity or paranasal sinuses, including involvement of the orbit, base of skull or intracranial cavity, cervical lymph nodes or distant metastatic sides |
Rationale for Treatment and Alternatives
Because of the rarity of esthesioneuroblastoma and its wide variety of clinical behavior, there is no definitive consensus regarding the optimal treatment. For small, low-grade tumors confined to the ethmoids, surgery alone appears to be an adequate method. Patients with locally advanced disease or high-grade tumors should receive aggressive treatment with combined modalities such as surgery, radiation therapy, and chemotherapy [38]. Most authors recommend en bloc resection, combined with radiation therapy [39, 40]. Local failure rates of 44 % in low-grade and 60 % in high-grade tumors and metastatic rates of 25 % in low-grade and 47 % in high-grade tumors are described [41]. A significantly lower recurrence rate with overall 5- and 10-year survival rates of 81 and 54.5 % in patients with response to neoadjuvant radiotherapy combined with chemotherapy has been reported [42]. At recurrence, either surgical and/or radiosurgical retreatment can lead to long lasting remissions in 42 % of patients [43, 44]. Thus close follow-up is recommended.
Radiosurgery for Esthesioneuroblastoma
Very few studies report on radiosurgical treatment in the primary situation; the limiting factors are usually the radiosensitivity of cranial nerves as discussed in Table 38.1.
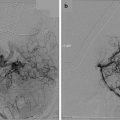
Walch et al. [45] reported on three patients with olfactory neuroblastoma treated with a combination of endoscopic surgery and Gamma Knife. Stereotactic radiosurgery was performed within the first 3 months of surgery. The maximum diameter of the tumors was approximately 24.3 mm and the marginal dose to the tumor varied from 16 to 34 Gy; 1–5 isocenters were used. Radiation-induced side effects were nasal discharge and crusts. One patient developed bilateral frontal chronic sinusitis, and in a second, endoscopic operation was necessary.
An Austrian group described the combined treatment of endoscopic surgery and radiosurgery for olfactory neuroblastoma [46]. Median marginal doses ranged from 15 to 34 Gy at a marginal isodose between 45 and 85 %. The maximum tumor volume treated with radiosurgery was approximately 20 cm3. The median follow-up period was 58 months. Observed radiosurgical side effects were mild and transient, such as cephalea and dizziness. No changes in mental status were observed. No new pathology of the optic pathway was described during follow-up.
Craniopharyngioma
Disease Pathophysiology
Craniopharyngiomas are benign tumors located at the base of the skull next to the pituitary gland. Differentiation between craniopharyngioma and pituitary can therefore sometimes be difficult on CT or magnetic resonance (MR) scans. Approximately 5–10 % of primary brain tumors are craniopharyngiomas. They typically occur in childhood as well as in the sixth to eighth decades [29]. Histopathologically, craniopharyngiomas are benign tumors arising from squamous cell remnants of the Rathke pouch during embryogenesis at the junction of the pituitary stalk and pituitary. Craniopharyngiomas present as a suprasellar lesion, frequently partially calcified and usually including an intrasellar component. These tumors are often composed of solid and cholesterol-rich cystic components. Cystic or solid components of this tumor extension may occur laterally into the middle or into the posterior cranial fossa. Symptoms relate to compression effects of the tumor due to its vicinity to pituitary gland, chiasm, optic nerves, and hypothalamic region. Locally, these tumors can produce signs and symptoms of increased intracranial pressure such as headache, drowsiness, or vomiting at the time of diagnosis and are due to hydrocephalus by obstruction of the foramen Monro by tumor parts within the third ventricle in 55–85 % of the patients [49]. Compression of the pituitary and hypothalamic region can produce antidiuretic hormone and growth hormone deficiency or obesity in children. Diabetes insipidus is present in approximately 10 % of the patients. Visual field defects and decreased vision due to compression of the optic chiasm and optic pathways are the initial symptoms in approximately 40–60 % of these patients [50, 51] (Table 38.4).
Table 38.4
Review of the literature for radiosurgery of craniopharyngioma
Author | N | Median follow-up (range) | Mean peripheral dose (range) | Local control (%)a | Morbidity |
|---|---|---|---|---|---|
Chung et al. [64] | 31 | 33 months (5–69)b | 12.2 Gy (9.5–16) | 87.2 | Visual field deficit (1 patient) |
Mokry [65] | 23 | 23 months (6–57) | 10.8 Gy (8–15) | 78.2 | None |
Ulfarsson et al. [67] | 21 | 42 months (6–348)b | 30 Gy (20–50)c | 36.4 | Visual field deficit (8 patients) |
Kobayashi et al. [68] | 98 | 66 months (6–148) | 11.5 Gy (NA) | 79.5 | Visual/endocrine (6 %) |
Iwata [62] | 44 | 40 (12–92) | 13–25 Gy (1–5 fractions) | 85 | Hypopituitarism (1 patient) |
Rationale for Treatment and Alternatives
The main treatment modality for craniopharyngiomas is surgery. Microsurgery allows complete tumor removal in 49–100 % of the patients with low morbidity and operative mortality [52–54]. After radical resection, 10-year progression-free survival rates between 60 and 93 % are reported [55, 56]. Treatment modalities include complete resection of the tumor with radiation therapy at the time of recurrence or subtotal resection followed by radiotherapy. The probability of complete tumor resection decreases with increasing tumor volume. Because of the proximity to critical normal structures and the relatively high association of radical surgery with visual loss and impaired hormone function requiring replacement therapy, many authors recommend less radical surgery (partial resection, biopsy, and aspiration of cystic contents) followed by radiation therapy or radiosurgery. With this strategy, local control rates of 70–83 % after 10 years are reported [57, 58] and assumed to be similar to complete surgical resection of the tumor [59]. Treatment-related toxicities after subtotal resection followed by radiotherapy include impairment of hormone function. Impairment of vision is reported for less than 10 % of all patients treated with the combination of subtotal resection and irradiation compared with up to 20 % after complete tumor resection [60]. Other side effects such as radionecrosis, radiation-induced malignancies, vascular morbidity, and cognitive decline occur less frequently [60, 61].
The major goal of radiotherapy treatment strategies is sparing of critical normal structures. Radiosurgery as well as intracavitary irradiation with stereotactically applied β[beta]-emitting radioisotopes maximize normal tissue sparing. The cystic nature of craniopharyngioma has led to trials of intracystic applications of β[beta]-emitting radioisotopes such as yttrium-90 or phosphorus-32. The use of radiosurgery has been reported in patients with minimal residual or recurrent disease. However, for patients with larger target volumes, tumors immediately abutting the optic apparatus and multiple cystic configured lesions, fractionated stereotactic radiotherapy should be preferred as excellent local control with minimal morbidity can be realized [62, 63].
Treatment Planning and Results for Radiosurgery
The target volume for craniopharyngiomas is narrowly defined to the tumor volume, including solid and cystic components. In cases with cyst aspiration or subtotal resection, it is important to cover the complete cyst wall. This technique can be used for selected patients with smaller tumors (<2 cm) not abutting critical structures such as the chiasm and the brain stem. Median doses to the margin of the tumor range from 9 to 16 Gy. Chung et al. recommend a margin dose of 12 Gy to induce satisfactory tumor response [64]. The main restriction with radiosurgery treatment is the tolerance dose of the neighboring visual pathway. The dose to the optic nerves and the chiasm should be kept below 8 Gy with single-dose techniques to avoid damage to these structures. Stereotactic radiosurgery has been used to treat small residual or recurrent tumors after surgical intervention.
Mokry et al. [65] treated 23 patients with Gamma Knife radiosurgery for craniopharyngioma and found no relevant morbidity. Ten patients had additional therapy with intracystic bleomycin before radiosurgery. Tumor progression was observed in 5 of 23 patients. They conclude that the best results might be obtained in monocystic tumors amenable to stereotactic drainage and intracystic bleomycin treatment. The Cologne group of Kickingrieder et al. summarizes their results on 53 patients with cystic craniopharyngiomas treated with stereotactically applied colloidal β-emitting radioactive sources. They concede few but notable severe side effects (hemiparesis and third nerve palsy) as well as suboptimal progression-free survival (79.4 ± 6.1, 72.4 ± 6.8, and 45.6 ± 8.7 % at 12, 24, and 60 months, respectively) [66].
After an average follow-up of 36 months, Chung et al. reported a tumor control rate of 87 % for 31 patients treated with Gamma Knife radiosurgery and a prescribed dose to the tumor margin from 9.5 to 16 Gy [64]. One patient developed a mildly restricted visual field. None of the patients showed additional endocrinologic impairment or neurologic deterioration related to radiosurgery. In a Swedish study, 21 patients were treated with Gamma Knife radiosurgery. They found a statistically significant difference between tumor progression and applied dose; a higher progression rate was found in patients treated with less than 6 Gy to the margin than in patients treated with a dose higher than 6 Gy. Four of these patients developed pituitary dysfunction [67]. In the literature, parenchymal injuries of the brain or second malignancies caused by radiotherapy are estimated to be less than 1–2 % [68].
Chordomas, Chondromas, and Chondrosarcomas
Disease Pathophysiology
Chondromas are rare benign tumors arising at the base of the skull, especially in the area close to the pituitary gland. It is a very-slow-growing tumor and might be present for a long time before causing any symptoms. Chondromas are composed of cartilage formed by the meninges and is usually attached to the dura mater. Surgical intervention might be the treatment of primary choice because of their usually well-defined margins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree