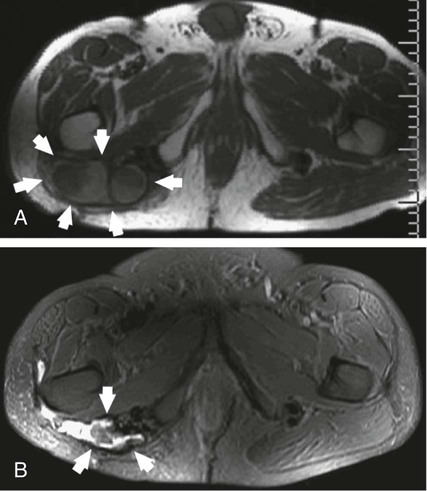
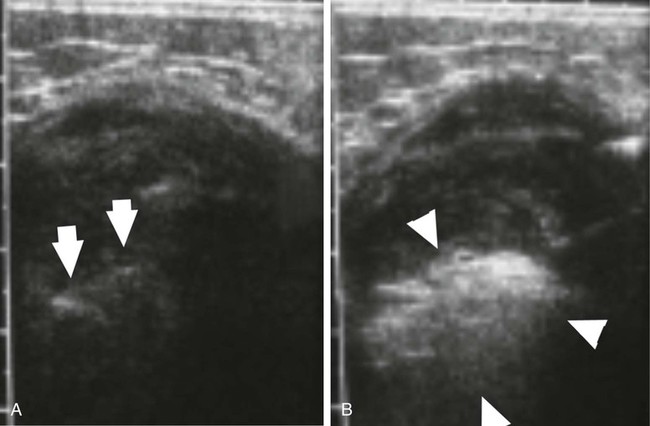
Desmoid tumors are locally infiltrative, and although they rarely metastasize, extensive morbidity and even mortality can occur from compression of adjacent organs. Growth of desmoids is characterized by extension along fascial planes with invasion of neurovascular structures (Fig. 162-1, A). Primary surgical treatment consists of wide excision or limb amputation. Recurrence develops in 25% to 70% of cases despite tumor-free margins, often within several months after resection. In approximately 90% of patients with positive margins, disease rapidly recurs within several months after resection. Percutaneous chemical ablation with 50% acetic acid or absolute ethanol has been used for in situ destruction of desmoid tumors (Fig. 162-2, B). The cytotoxic effects of acetic acid are derived from protein desiccation, lipid dissolution, and collagen extraction, whereas those of ethanol are attributed to cytoplasmic dehydration, denaturation of cellular proteins, and small vessel thrombosis. These effects culminate in coagulative necrosis indistinguishable from thermal ablation techniques. Instances of significant tumor reduction have been observed after even a single session of chemical ablation, suggesting that other mechanisms such as apoptosis or immune-modulated tumor destruction may also play a role in tumor regression. Indications for percutaneous chemical ablation include: • Enlarging or symptomatic desmoid tumor (pain, limitation of limb motion), or recurrent tumor after previous surgical excision • Percutaneously accessible without traversing lung, bowel, or other vital organs • Lack of invasion of a major neurovascular structure or a vital organ within the intended treatment area • Desmoid tumor 10 cm or less in maximum diameter by RECIST (Response Evaluation Criteria in Solid Tumors) criteria After positioning of one to three needles within the center of the desmoid tumor, acetic acid is infused. Real-time ultrasound monitoring is preferable. It is vital that an extremely slow rate of infusion be used. For this reason, a calibrated 1-mL Luer-Lok syringe is helpful. This enables the operator to accurately monitor the rate and pressure of injection. Generally, acetic acid is injected at a rate of approximately 0.1 mL per 15 to 20 seconds. A rapid burst of the agent is undesirable; rather, the technique relies on steady and gradual dispersal of the agent. As acetic acid penetrates through the tumor, the baseline hypoechoic echotexture of the tumor will become immediately hyperechoic (see Fig. 162-2). Further injection is performed as the needle is slowly withdrawn away from the central core of the tumor in an attempt to render the inner aspect of the tumor as uniformly echogenic as possible. The volume of acetic acid during an individual treatment session is divided between the additional infusion needles. In total, no more than 10 mL of acetic acid is injected in a single treatment session. Even large tumors may require a much smaller volume than this to produce a large area of echogenicity in the tumor. Because desmoids are not encapsulated, great care must be taken to not allow extravasation of the agent through the interstices of the tumor. Care is also taken to ensure distribution of acetic acid in the peripheral margins. If the periphery of the tumor becomes hyperechoic during the injection, the infusion is stopped, and the needle is either repositioned or withdrawn. • Real-time ultrasound monitoring during chemical ablation is preferable. • A single intravenous dose of a broad-spectrum antibiotic (e.g., cefazolin, vancomycin) is given. • Conscious sedation is achieved with intravenous fentanyl citrate and midazolam. • One to three needles (Bernardino, Chiba) are positioned within the center of the desmoid tumor under local anesthesia. • Once the needles are positioned, acetic acid is injected through a single needle at a rate of approximately 0.1 mL per 15 to 20 seconds via a calibrated 1-mL Luer-Lok syringe. • The desmoid tumor will change from a hypoechoic appearance to a bright echotexture during injection. • The injection is performed to a sonographic endpoint to render the central two thirds of the tumor as uniformly echogenic as possible. • Further injection is performed as the needle is slowly withdrawn away from the central core of the tumor in an attempt to render the inner aspect of the tumor as uniformly echogenic as possible. • The needle is removed after aspiration to minimize seepage of acetic acid during tumor withdrawal. • The same process is repeated with the remaining needle or needles. • In total, no more than 10 mL of 50% acetic acid is injected during a single treatment session. • When needles are reinserted during subsequent treatment sessions, brownish sterile fluid from necrotic tumor tissue as a result of previous treatment may be encountered. This fluid is aspirated before injection of additional acetic acid.
Chemical and Thermal Ablation of Desmoid Tumors
Chemical Ablation
Indications
Technique
Anatomy and Approach
Technical Aspects
Chemical and Thermal Ablation of Desmoid Tumors