Cholangiocarcinoma
Todd M. Blodgett, MD
Alex Ryan, MD
Omar Almusa, MD
Key Facts
Terminology
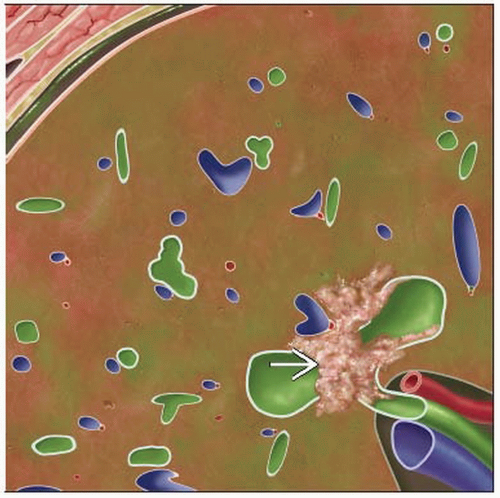
Malignancy that arises from ductular epithelium of intrahepatic biliary tree, extrahepatic bile ducts
Imaging Findings
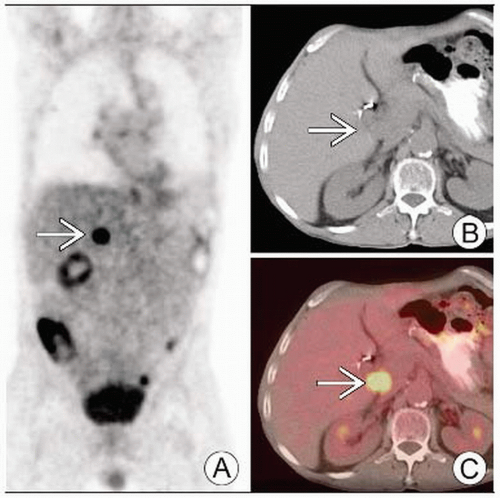
PET: Hypermetabolic activity corresponding to primary tumor in liver, extrahepatic metastatic disease
Ultrasound (US), CT, MR: For hilar lesions (Klatskin tumor), bile duct obstruction with small central mass
Extrahepatic tumors (87-92% of CC): Proximal, middle, distal ductal tumors
PET for staging distant metastases; characterizing peripheral CC
Delayed enhancement with increasing attenuation seen in up to 74% of lesions, usually ↑ CT sensitivity/specificity
Hilar CC: Low FDG activity with focal nodular or linear branching pattern
Top Differential Diagnoses
Hepatocellular Carcinoma (HCC)
Primary Sclerosing Cholangitis (PSC)
Focal Nodular Hyperplasia (FNH)
Cavernous Hemangioma
Pancreatic Carcinoma
Clinical Issues
Obstructive jaundice (90%)
Diagnostic Checklist
Ability of PET to detect distant metastases alters surgical management (reportedly up to 30% of cases)
PET sensitivity for distant mets 65-70%
PET sensitivity for regional or hepatoduodenal mets is approximately 13%
TERMINOLOGY
Abbreviations and Synonyms
Cholangiocarcinoma (CC), Klatskin tumor, malignant bile duct tumor
Definitions
Malignancy that arises from ductular epithelium of intrahepatic biliary tree and extrahepatic bile ducts
Note: Gallbladder cancer 9x more common than CC
Klatskin tumor: Perihilar cholangiocarcinoma involving bifurcation of hepatic duct; accounts for more than 70% of all bile duct cancers
IMAGING FINDINGS
General Features
Best diagnostic clue
PET: Hypermetabolic activity corresponding to primary tumor in liver, extrahepatic metastatic disease
Ultrasound, CT, MR: Bile duct obstruction w/small central mass suggests hilar lesion (Klatskin tumor)
Location
Extrahepatic tumors (87-92% of CC): Proximal, middle, distal ductal tumors
Extrahepatic tumor at bifurcation of proximal common hepatic duct = Klatskin tumor
Intrahepatic tumors (8-13% of CC) arise from small ducts
Nodular or papillary type is most common in distal duct and periampullary region
Intrahepatic tumors have tendency for perineural spread, but spread to liver, peritoneum, lung is extremely rare
Extrahepatic tumors spread to celiac nodes in ˜ 16% of cases
Size
Peripheral lesions are usually larger, measuring 5-20 cm at presentation
More central lesions (Klatskin) smaller at diagnosis
Morphology
Variable
Most intrahepatic CC present as mass, whereas 90% of extrahepatic CC reveal diffusely infiltrating growth pattern
Imaging Recommendations
Best imaging tool
CT: Staging regional/distant metastases; similar to US for demonstrating ductal dilation, large mass lesions
MRCP/ERCP: Sensitivity of 71-81% for detecting tumor in malignant stenoses, particularly central lesions
PET for staging distant metastases and characterizing peripheral CC
ERCP with brush cytology, DNA analysis, and serum analysis of CA 19-9 and CEA for initial workup
Have been shown to increase sensitivity significantly
Diagnosis of CC, especially in primary sclerosing cholangitis (PSC), may remain uncertain until invasive and aggressive approaches such as exploratory laparotomy provide biopsy
Protocol advice
Delayed PET imaging at ˜ 120 minute time point shown to better discriminate tumor from inflammation
Delayed imaging helps differentiate tumor from background liver activity
CT Findings
NECT
Mass predominantly hypoattenuating with irregular margins
Intrahepatic biliary duct (IHBD) dilation common with obstruction
Larger peripheral lesions may be isodense with central low attenuation and scarring
Central and satellite lesions
Hilar masses often not visible on NECT
IHBD dilation = clue
Capsular retraction may reveal intrahepatic tumor
Large common duct (extrahepatic) masses may be identified on NECT
CECT
Solitary, small, well-demarcated tumors are difficult to differentiate from primary hepatocellular carcinoma (HCC)
Arterial phase: Peripheral CC seen as intrahepatic mass showing early peripheral rim enhancement and progressive patchy central enhancement
Portal phase: Portal vein invasion, ductal wall thickening with minimal enhancement, and portal lymphadenopathy
Delayed phase
Enhancement with increasing attenuation seen in up to 74% of lesions, usually ↑ CT sensitivity/specificity
Persistent tumor enhancement due to fibrous stroma
Low reported sensitivity for small hilar lesions (approximately 50%)
Regional lymph node spread rarely detected (24-40% of cases)
Nuclear Medicine Findings
FDG PET
Primary uses
Identification of new lesions
Evaluation of metabolic activity and associated malignancy
Characterization of response to neoadjuvant therapy
Detection of lesions in liver that are not suspected on US or MR in up to 50% of patients
Peripheral CC: Intensely hypermetabolic activity, may be ring-shaped
Hilar CC: Low activity with focal nodular or linear branching pattern
Lower FDG uptake may be related to tumor size or arrangement of fibrous stroma and mucin pool in tumor
Can be difficult to discriminate between extrahepatic tumor itself and FDG-accumulating lymph nodes in perihilar region
Extrahepatic CC may have low uptake due to loosely connected cell nests and poor detection with PET due to infrequency of evident mass formation
PET sensitivity
61-90% for primary CC
85% for nodular CC
18% for infiltrating CC
65-70% for distant metastases
Only 13% for regional or hepatoduodenal mets
False negatives are seen with mucinous adenocarcinomas (rare)
False positives are seen due to foci of inflammation (e.g., intrahepatic stone)
Uptake likely to be seen along tract of biliary stents
Primary sclerosing cholangitis (PSC)
PET can be used to discriminate between PSC with and without CC
Not reliable for early diagnosis of CC in patients with PSC
Liver in patients with PSC may have ↑ background signal than those of healthy control patients
PET/CT
Allows better identification of non-FDG avid tumors & carcinomatosis and helps distinguish stent-related uptake from malignant disease
Shown to change oncological management in up to 17% of patients
No diagnostic advantage over CECT in detection of intrahepatic CC or primary tumor site of extrahepatic CC
Generally cost-effective method, avoids unnecessary surgery
Hepatobiliary scintigraphy: Focal photopenic lesion
Tc-99m sulfur colloid: Focal photopenic lesion
Ga-67 scintigraphy: Variable Ga-67 uptake
DIFFERENTIAL DIAGNOSIS
Hepatocellular Carcinoma (HCC)
NECT typically shows an iso- or hypodense mass
Shows early enhancement on CECT (vs. late enhancement in CC)
Variable FDG uptake with ˜ 50% having little/no FDG uptake due to ↑ glucose-6-phosphatase
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree