Chapter Outline
Acute Acalculous Cholecystitis
Complications of Acute Cholecystitis
Other Conditions Related to Gallstones
This chapter provides a review of the imaging assessment of gallbladder and biliary calculi. Cholecystitis, both acute and chronic, and associated complications are discussed. Other disorders related to gallstones, including spilled gallstones after laparoscopic surgery, Mirizzi syndrome, and gallstone ileus, are also reviewed. The chapter concludes with a discussion of the spectrum of noninflammatory gallbladder conditions referred to as the hyperplastic cholecystoses.
The imaging evaluation of cholelithiasis and associated complications has evolved significantly in the past decade. Cholescintigraphy and sonography continue to serve as the primary imaging modalities for the initial assessment of most suspected gallbladder disorders. Computed tomography (CT) and magnetic resonance imaging (MRI), however, have proved to be of considerable value in certain circumstances. These may be performed for initial evaluation or serve as an important adjunct to other imaging modalities when further information is required. The role of CT and MRI in the evaluation of cholecystitis, choledocholithiasis, and the hyperplastic cholecystoses is discussed.
Cholelithiasis
Pathogenesis and Epidemiology
The prevalence of gallstones varies with age and gender. Gallstones are present in an estimated 10% to 15% of men and 20% to 40% of women older than 60 years. In general, the risk for stones increases with history of childbearing, estrogen replacement therapy, oral contraceptive use, and obesity. Stones are also associated with hypertriglyceridemia, Crohn’s disease, and parenteral hyperalimentation. Gallstones are symptomatic in 20% to 30% of patients; biliary pain or colic is the most common symptom. This is most often related to impaction of a stone in the cystic duct. The most common acute complications of gallstones are acute cholecystitis, acute pancreatitis, and ascending cholangitis. Chronic complications include chronic cholecystitis, Mirizzi syndrome, cholecystenteric fistula, and gallstone ileus.
Gallstones are composed mainly of cholesterol, bilirubin, and calcium salts with smaller amounts of protein and other materials, including bile acids, fatty acids, and inorganic salts. Stones form when there is supersaturation of various bile components. Lithogenic bile usually results from increased biliary cholesterol output, but decreased bile acid synthesis or a combination defect may play a role in stone development. Biliary dysmotility and prolonged intestinal transit may also be contributing factors.
In Western countries, cholesterol is the principal constituent of more than 75% of gallstones. Many stones are more than 80% cholesterol with smaller amounts of calcium bilirubinate. Pure cholesterol stones contain more than 90% cholesterol and are relatively uncommon, accounting for less than 10% of biliary calculi. Pigment stones contain less than 25% cholesterol and are relatively uncommon, representing 10% to 25% of gallstones in North America. These stones consist of calcium salts of bilirubin and are categorized as black or brown pigment stones. Black pigment stones consist of polymers of bilirubin with large amounts of mucin glycoproteins. These are more common in patients with cirrhosis or chronic hemolytic anemias in whom biliary excretion is increased. Brown pigment stones are made up of calcium salts of unconjugated bilirubin, with variable amounts of protein and cholesterol, and are more commonly associated with bacterial infection. The deconjugation of bile by bacterial enzymes is also considered an etiologic factor.
Imaging
Abdominal Radiography
Only about 15% to 20% of gallstones are calcified enough to be visualized on conventional abdominal radiographs ( Fig. 77-1 ). Therefore, abdominal radiographs have a limited role in the detection of gallstones. Oral cholecystography was introduced in the 1920s and for many years was the primary modality for evaluation of gallbladder disease, including stones. However, this technique has largely been replaced by sonography.

Ultrasound
Sonography is now considered the imaging tool of choice for the detection of gallstones, with a reported accuracy of 96%. However, recent advances in sonographic technology resulting in improved spatial resolution may allow even higher diagnostic accuracy. Other advantages of sonography include the ability to perform studies at the bedside and lack of ionizing radiation. Sonography can also evaluate other structures in the right upper quadrant when alternative diagnoses are under consideration.
At sonography, a gallstone appears as a highly reflective echo that is generally mobile and associated with posterior acoustic shadowing ( Fig. 77-2 ). Both mobility and acoustic shadowing are important features that help differentiate gallstones from other echogenic foci in the gallbladder lumen, such as sludge or solid masses. An aggregate of sludge or sludge ball (also referred to as tumefactive sludge) may be mobile but will not cast an acoustic shadow ( Fig. 77-3 ). A solid mass will not be mobile and will not shadow and may exhibit vascularity on color Doppler interrogation.
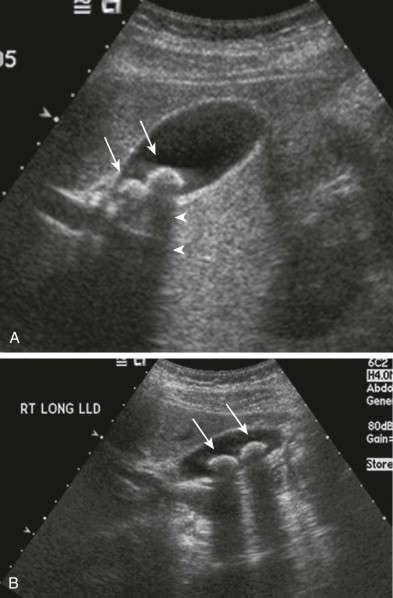

Very small stones may not always cast a shadow. Ex vivo studies have demonstrated that calculi larger than 3 mm produce acoustic shadowing regardless of composition. The demonstration of a posterior acoustic shadow, however, also depends on transducer frequency and beam width. In a study by Grossman, when a 5-MHz transducer was used, 0.2- to 0.3-cm stones were associated with shadowing, whereas when a 2.25-MHz transducer was used, only stones measuring at least 0.4 cm cast a shadow. Using a tissue phantom, Filly and associates found that acoustic shadowing was present when the stone was at or near the center of the beam but not at the periphery. These studies demonstrate that to maximize detection, the highest frequency transducer possible should be used with the focal zone placed at the level of the stone. In recent years, tissue harmonic imaging has been shown to improve the detection rate of gallbladder calculi ( Fig. 77-4 ).

Gallstones may not be detected at sonography if they are small or hidden behind a gallbladder fold or in the cystic duct, if intraluminal sludge is present, or if the examination is technically suboptimal related to the patient’s body habitus or the operator’s inexperience. Chintapalli and colleagues studied 946 patients with surgically proved gallstones. In 98.7%, preoperative sonography showed single or multiple echogenic foci with or without acoustic shadowing in the gallbladder for a false-negative rate of 1.3%. In the patients with false-negative examinations, sonographic findings were consistent with polyps (five cases), sludge, or both, and the ultrasound was interpreted as normal in one patient. Missed stones were 5 mm or smaller in 10 patients and smaller than 1.0 cm in all 12 patients. Stones located in the gallbladder neck or trapped behind a fold may be overlooked when the patient is evaluated only in the supine position. Also, small stones may become more conspicuous when the patient is evaluated in the decubitus or upright position as they produce a shadow only when they are imaged in aggregate ( Fig. 77-5 ). Therefore, it is always important to evaluate the patient in more than one position in assessing for the presence of gallstones. A follow-up study may be suggested if the clinical index of suspicion is high and the initial examination is negative.

If the gallbladder is contracted and the lumen is filled with shadowing stones, a high-amplitude echo results that is usually linear or curvilinear in configuration and associated with posterior acoustic shadowing. Careful observation will demonstrate a perceivable gallbladder wall separate from the intraluminal stones, sometimes facilitated by the use of a high-resolution linear transducer ( Fig. 77-6 ). This appearance has been called the wall-echo-shadow sign. The main differential diagnostic considerations are a porcelain gallbladder, with calcification in the gallbladder wall, and air in the gallbladder, such as in emphysematous cholecystitis. Correlation with abdominal radiography or CT can be helpful as a problem-solving tool ( Fig. 77-7 ).


Computed Tomography
The ability to detect gall stones at CT depends on differing density of the stone with respect to bile. The reported sensitivity of CT for detection of gallstones is approximately 75%. Calcified stones are readily identified as they are denser than bile ( Fig. 77-8A ). Stones with high concentration of cholesterol may also be readily identified as these stones are less dense than bile ( Fig. 77-8B ). When stones degenerate, nitrogen gas may collect in central fissures and create the Mercedes-Benz sign. This may be the only visualized evidence of a gallstone and appear as a focal collection of gas in the nondependent gallbladder lumen ( Fig. 77-8C ). Noncalcified pigment stones are soft tissue attenuation density ( Fig. 77-8D ). Many stones are composed of a mixture of calcium, bile pigments, and cholesterol and may be similar in density to bile and therefore not visible at CT ( Fig. 77-9 ). The size of the stone is also an important factor determining if a stone is visible at CT. Small stones are frequently missed unless the density differs markedly from bile. In one series, only 78.9% of patients with stones demonstrated at sonography were found to have stones at CT. Most stones missed prospectively were faintly calcified and were retrospectively detected only as subtle defects within the gallbladder. A phantom study demonstrated that the sensitivity of CT for detection of gallstones varies with peak voltage and is highest when CT is performed at 140 kVp rather than at lower voltage settings.


Magnetic Resonance Imaging
On T2-weighted MRI, gallstones are manifested as signal voids in the high signal intensity bile ( Fig. 77-10 ). Cholesterol stones are generally isointense or hypointense on T1-weighted images, whereas pigment stones have been shown to have high signal intensity on T1-weighted images. This signal hyperintensity is caused by the presence of metal ions in the pigment stones, shortening the T1 relaxation time. Another study of gallstone appearance at MRI also correlated signal intensity with chemical composition. T2-weighted central high signal intensity corresponded to fluid-filled clefts in the stones, whereas central and peripheral high signal areas on T1-weighted images corresponded to fluid clefts as well as regions high in copper content. Other intraluminal filling defects that may mimic gallstones on T2-weighted images include tumor, blood clot, and gas bubbles.

Biliary Sludge
Pathogenesis
Biliary sludge, also referred to as microlithiasis, biliary sand or sediment, pseudolithiasis, and microcrystalline disease, is a suspension of bile and particulate material in the gallbladder. The chemical composition consists of various proportions of calcium bilirubinate and cholesterol monohydrate crystals and gallbladder mucus. The proposed pathogenesis is similar to that of gallstones. A combination of impaired gallbladder motility and alteration in nucleation factors leads to the formation of sludge, with additional precipitate aggregation resulting in gallstone formation. Clinical conditions that increase sludge formation include fasting, pregnancy, total parenteral nutrition, and critical illness. Sludge may resolve, have a cyclic pattern of appearance and disappearance, or progress to stone formation. Although patients may generally be asymptomatic, symptoms can include biliary pain, cholecystitis, cholangitis, or pancreatitis. Treatment is symptom directed.
Imaging
At sonography, sludge typically appears as low-level echoes that layer dependently within the gallbladder lumen ( Fig. 77-11 ). When the gallbladder lumen is entirely filled with sludge, this yields an appearance referred to as hepatization of the gallbladder. In this situation, the gallbladder assumes the same echotexture as the liver parenchyma ( Fig. 77-12A, B ). The use of color Doppler imaging is important to exclude other more significant disease, such as intraluminal soft tissue masses. If findings are equivocal and there is suspicion for a possible intraluminal mass, MRI with contrast and subtraction images can be helpful ( Fig. 77-12C, D ). An aggregate of intraluminal sludge can also mimic a soft tissue density mass (tumefactive sludge). As discussed, gravity dependence should be demonstrated to differentiate sludge from a gallbladder polyp or mass ( Fig. 77-13 ). Doppler demonstration of vascularity within the abnormality confirms the presence of a soft tissue mass. However, absence of demonstrable vascularity is less helpful, and short-interval follow-up examination or assessment with precontrast and postcontrast CT or MRI may be indicated.



Acute Cholecystitis
Pathogenesis and Epidemiology
Acute cholecystitis occurs in approximately one third of patients with gallstones. It results from persistent obstruction of the cystic duct or gallbladder neck, with distention of the gallbladder and increased intraluminal pressure. Inflammation of the gallbladder mucosa may result from chemical injury caused by bile salts or superimposed infection. If left untreated, the inflammation eventually progresses to involve all layers of the gallbladder wall and may lead to necrosis, gangrene, and gallbladder perforation. In most patients, acute cholecystitis is associated with gallstones.
Acute cholecystitis is the most common cause of right upper quadrant pain, and the primary mode of treatment is laparoscopic cholecystectomy. Clinical findings include fever, right upper quadrant pain, and elevated white blood cell count. Approximately one third of patients have a clinically positive Murphy sign, which refers to focal tenderness over the gallbladder on inspiration. However, the clinical manifestations may overlap considerably with other intra-abdominal processes not related to the biliary tract, such as acute hepatitis, primary liver disease including abscess and neoplasm, peptic ulcer disease, and pancreatitis. Also, clinical findings in the elderly or critically ill patient may be more subtle. Approximately one third of patients with the presumptive diagnosis of acute cholecystitis will not have acute cholecystitis on follow-up, and 20% to 25% of patients who have surgery for acute cholecystitis will ultimately have a different diagnosis. In a series of 52 patients with right upper quadrant pain thought to have acute cholecystitis, acute cholecystitis was confirmed in 34.6%, chronic cholecystitis was confirmed in 32.7%, and 32.7% had normal gallbladders. Therefore, imaging evaluation is critical to provide prompt diagnosis and appropriate intervention.
In 2007, the Tokyo Guidelines that describe the diagnostic and severity assessment criteria for acute cholecystitis were published, and these were recently updated in 2013. According to these guidelines, the diagnostic criteria for acute cholecystitis are one local sign of inflammation (Murphy sign; mass, pain, or tenderness in right upper quadrant), one systemic sign of inflammation (fever, elevated C-reactive protein level, elevated white blood cell count), and confirmatory imaging findings. Cholecystitis severity is classified as mild, moderate, or severe (stages I, II, III, respectively). Mild cholecystitis is defined as cholecystitis with mild inflammatory changes adjacent to the gallbladder without organ dysfunction. Moderate cholecystitis includes elevated white blood cell count, palpable tender mass in the right upper quadrant, duration of symptoms longer than 72 hours, and marked local inflammation. Severe cholecystitis is defined as cholecystitis combined with multiple organ dysfunction. Radiologic findings may have an important impact on how a patient with acute cholecystitis is treated once the diagnosis is established. In a patient with sepsis and organ failure, percutaneous drainage with delayed cholecystectomy may be the best option. Patients without severe inflammation may be best managed with early laparoscopic cholecystectomy. Up to 30% of patients treated laparoscopically may undergo conversion to an open procedure, usually in the setting of severe complicated cholecystitis with dense pericholecystic inflammation and adhesions. Preoperative imaging may also help predict which patients are likely to require conversion to an open procedure.
Imaging
The relative role of ultrasonography versus cholescintigraphy with technetium-tagged iminodiacetic acid (IDA) and IDA-like agents for the diagnosis of acute cholecystitis has been extensively addressed in the literature. In an early study published in 1982, the accuracy of scintigraphy with IDA compared with sonography showed similar excellent results in 91 patients with suspected acute cholecystitis, with accuracy of ultrasound of 88% and of scintigraphy of 85%. A study in 1983 of 194 patients showed that sensitivity of both modalities was high, but the specificity of ultrasound was lower at 64%, with a positive predictive value of 40%. The sonographic Murphy sign, however, was not evaluated in this study, and there was no correlation with clinical data. More recent studies comparing cholescintigraphy and sonography for diagnosis of acute cholecystitis showed an accuracy of scintigraphy of 91% versus 77% for sonography and sensitivity of scintigraphy of 90.9% versus 62% for ultrasound. Most recently, a systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis was performed. Fifty-seven studies were included with evaluation of 5859 patients. The sensitivity and specificity were 96% and 90% for cholescintigraphy compared with 81% and 83% for ultrasound.
Although it is possibly somewhat less sensitive and specific for the diagnosis of acute cholecystitis compared with cholescintigraphy, ultrasound does have the advantages of being readily available and rapidly performed, involving no radiation, and allowing detection of cholecystitis-related complications as well as alternative diagnoses. Ultrasound can confirm the diagnosis of acute cholecystitis and distinguish it from chronic cholecystitis with an accuracy of 95% to 99%. The disadvantages of radionuclide scintigraphy include the time to perform the examination (up to 4 hours) and the inability to evaluate for nonbiliary conditions. Furthermore, false-positive results can occur in patients with high bilirubin levels and severe intercurrent illnesses. False-negative results, however, are rare.
Depending on the clinical circumstances, a combination of both cholescintigraphy and sonography may be required to make a definitive diagnosis of acute cholecystitis. The American College of Radiology appropriateness criteria for evaluation of patients with right upper quadrant pain, most recently updated in 2010, score ultrasound higher than cholescintigraphy for evaluation of the patient with fever, elevated white blood cell count, and positive Murphy sign and state that cholescintigraphy should generally follow ultrasound on the basis of ultrasound findings. If there is pain but no fever or leukocytosis, ultrasound is given the highest score and may allow detection of stones and bile duct obstruction. If there are only gallstones and pain, cholescintigraphy, CT, and MRI are given equal scores. CT and MRI may allow detection of other causes of pain, improved detection of choledocholithiasis, and further evaluation when it is required, such as when complications are suspected (discussed further later).
Cholescintigraphy
The hepatic parenchyma is normally observed within 1 minute, with peak activity at 10 to 15 minutes. The bile ducts are usually visible within 10 minutes, and the gallbladder should appear within 1 hour if the cystic duct is patent ( Fig. 77-14A ). If the gallbladder has not been defined, imaging should be carried out farther, up to 4 hours. Prompt biliary excretion of tracer without demonstration of the gallbladder is the hallmark of acute cholecystitis ( Fig. 77-14B ). As noted before, false-positive results may occur in patients with abnormal bile flow because of liver disease or a prolonged fast with distended sludge-filled gallbladder. Also, delayed gallbladder filling can occur in the setting of chronic cholecystitis. If the gallbladder is not visualized after 1 hour and there is visualization of the biliary tree and duodenum, intravenous morphine may be administered, which causes spasm of the sphincter of Oddi, raising pressure within the bile ducts and increasing the likelihood of bile flow into the gallbladder. If the patient has been fasting for more than 24 hours, an oral fatty meal or intravenous cholecystokinin may be administered, resulting in gallbladder contraction so that the gallbladder can empty and then refill.

Ultrasound
The ultrasound findings in acute uncomplicated cholecystitis are well described and include gallstones, sonographic Murphy sign, gallbladder distention, wall thickening, and pericholecystic fluid ( Figs. 77-15 and 77-16 ). The sonographic Murphy sign refers to maximum tenderness during compression with the ultrasound transducer placed directly over the gallbladder. Of these findings, the first two are considered the most specific. In a study by Ralls and coworkers, the sonographic Murphy sign and the presence of gallstones had a positive predictive value of 92% for the diagnosis of acute cholecystitis. It is cautioned that the sonographic Murphy sign may be blunted if the patient has received pain medication before ultrasound evaluation. Furthermore, impaired mental status may preclude evaluation of this sign. Last, the sonographic Murphy sign may be absent in the setting of gangrenous cholecystitis (see later). If it can be demonstrated that a stone is impacted in the gallbladder neck or cystic duct, this is also an important finding that increases the likelihood of acute cholecystitis ( Fig. 77-15B ). To determine that a stone is impacted in the gallbladder neck or cystic duct requires evaluating the patient in the left lateral decubitus or upright position to assess for mobility of the stone.


Less specific ultrasound findings of acute cholecystitis include gallbladder distention, wall thickening, pericholecystic fluid, and the presence of other intraluminal material such as sludge. In most cases of acute cholecystitis, the gallbladder will be distended. An exception to this occurs when acute cholecystitis complicates chronic cholecystitis, when there may be mural fibrosis impeding distention. Also, if there has been free perforation of the gallbladder, it may appear completely collapsed (discussed further later). Diffuse gallbladder wall thickening, measuring more than 3 mm, is present in 50% to 75% of patients with acute cholecystitis but may also be associated with chronic inflammation. Furthermore, gallbladder wall thickening may be associated with many other conditions, including liver disease such as acute hepatitis ( Fig. 77-17 ), ascites, hypoalbuminemia, and alcoholism as well as congestive heart failure, acquired immunodeficiency syndrome (AIDS), and sepsis. In patients with AIDS, cholangiopathy may be related to infection with organisms such as Cryptosporidium. Other causes include adenomyomatosis and gallbladder neoplasm. In acute cholecystitis, wall thickening is generally diffuse; if it is more focal, a complication such as gangrenous change or another cause, such as neoplasm, should be considered. Pericholecystic fluid is generally associated with more severe cholecystitis and may be associated with perforation or abscess formation. However, this may also be nonspecific, especially in the setting of generalized ascites. Other entities that clinically can mimic acute cholecystitis, such as peptic ulcer disease and pancreatitis, may also be associated with pericholecystic fluid. Sludge, related to bile stasis, can develop in patients with acute cholecystitis due to gallbladder obstruction.

The role of color and power Doppler evaluation as an adjunct to gray-scale imaging in the diagnosis of acute cholecystitis is somewhat controversial. Although there may be overlap between findings in acute and chronic cholecystitis, there may be a potential role for Doppler evaluation of the inflamed gallbladder ( Fig. 77-18 ).

Computed Tomography
Although it is not the first-line imaging modality for the evaluation of suspected acute gallbladder disease, the role of CT in the evaluation of the patient with acute abdominal pain continues to expand. The cause of the patient’s symptoms may initially be unclear because of nonspecific clinical findings. In this setting, CT may be the initial study performed as it allows more comprehensive evaluation of the abdomen and pelvis and can identify other acute inflammatory processes that may simulate acute cholecystitis. Therefore, it is important to become familiar with the spectrum of CT findings in acute cholecystitis. CT is also a useful adjunct to ultrasound when ultrasound findings are equivocal or when a complicated form of cholecystitis is suspected (see later). In a retrospective study at the author’s institution, the overall sensitivity, specificity, and accuracy of CT for the diagnosis of acute cholecystitis were 91.7%, 99.1%, and 94.3%, respectively.
The CT findings of acute cholecystitis include gallstones, gallbladder distention, thickening of the gallbladder wall, pericholecystic inflammation, and fluid ( Fig. 77-19 ). The CT findings of acute cholecystitis have been divided into major and minor criteria. Major findings include calculi, mural thickening of the gallbladder, pericholecystic fluid, and subserosal edema. Minor findings are gallbladder distention and sludge. CT is limited with respect to detection of gallstones as only up to 75% are visualized. Of these signs, the presence of pericholecystic inflammatory change is believed to be the most specific. Gallbladder wall thickening on CT is a nonspecific finding and may be secondary to a broad spectrum of both inflammatory and neoplastic disorders. Gallbladder carcinoma tends to cause greater mural thickening than benign disorders do.

On occasion, it may be difficult to differentiate gallbladder wall thickening from pericholecystic fluid. Pericholecystic fluid tends to be more focal and irregularly marginated, whereas mural thickening is concentric. Punctate foci of contrast enhancement corresponding to enhancing vessels within the gallbladder wall may be observed. A target appearance corresponds to an inner layer of enhancing mucosa and an outer layer of enhancing serosa with hypoattenuating submucosal edema between the two.
An ancillary CT finding of gallbladder inflammation is increased contrast enhancement in the liver parenchyma adjacent to the gallbladder ( Fig. 77-20 ). This finding results from hepatic arterial hyperemia secondary to gallbladder inflammation. Increased density of the gallbladder wall has been described as a finding on unenhanced CT, found in approximately 51% of patients. This is found in patients with mucosal hemorrhage and necrosis and may be a predictor of gangrenous change.

A study evaluating the use of 64-section helical CT for quantitative and qualitative assessment of acute cholecystitis found that pericholecystic fat stranding, mural stratification, pericholecystic hypervascularity, spontaneous hyperattenuation of the gallbladder wall, short (≥32 mm) and long (≥74 mm) gallbladder axis enlargement, and gallbladder wall thickening (≥3.6 mm) were the most discriminating and independent variables for the CT diagnosis of acute cholecysitis. Comparative studies assessing accuracy of CT versus ultrasound for diagnosis of acute cholecystitis are limited. In a study by van Randen and associates that compared CT and ultrasound in 52 patients with acute cholecystitis, diagnostic accuracy did not differ significantly between the two modalities. However, in their meta-analysis, Kiewiet and colleagues cautioned that additional studies evaluating the diagnostic accuracy of CT for acute cholecystitis are needed and that there is currently not enough evidence to support indiscriminate use of this modality in patients suspected of having acute cholecystitis.
Brook and associates reviewed 14 cases of misdiagnosis of acute cholecystitis at both ultrasound and CT in their practice during a 5-year period. Eight cases of misdiagnosis involved ultrasound and six cases involved CT. Three cases of overcall on ultrasound included cases of gallbladder wall edema that ultimately were diagnosed as hepatitis, sepsis, and chronic cholecystitis. In none of these patients was the gallbladder distended. Therefore, if the gallbladder wall is thickened but there is no distention, an alternative diagnosis should be considered. Two cases of undercall at ultrasound were in patients in the intensive care unit, and two cases were due to lack of wall edema. In an additional patient, diagnosis was delayed because the ultrasound report was not definitive. All of the misdiagnoses at CT were due to undercalls; in one patient, lack of intra-abdominal fat hindered recognition of pericholecystic fat stranding. In another patient, pericholecystic increased arterial enhancement in the liver was mistaken for fatty sparing, and in an additional patient, wall edema and mucosal enhancement were mistaken for a calculus.
Magnetic Resonance Imaging
Like CT, MRI is usually used in the setting of suspected acute cholecystitis when other imaging findings are equivocal or the clinical setting is ambiguous. Magnetic resonance cholangiopancreatography (MRCP) is sensitive in the diagnosis of choledocholithiasis, which is particularly important if laparoscopic cholecystectomy is performed. In addition to gallstones, MR readily demonstrates gallbladder wall edema, which is manifested as high signal intensity on T2-weighted images. In one series, pericholecystic high signal intensity on single-shot fast spin-echo (SSFSE) images had an overall accuracy of 89%, specificity of 79%, positive predictive value of 87%, and negative predictive value of 85% for the diagnosis of acute cholecystitis. Loud and associates found contrast-enhanced T1-weighted images useful for the diagnosis of acute cholecystitis. Patients with surgically proved acute cholecystitis had greater than 80% contrast enhancement of the wall, which helped differentiate acute from chronic cholecystitis. A transient increase in hepatic enhancement may be seen around the gallbladder in 70% of cases during the arterial phase of enhancement and, as with CT, may be an important indicator of acute gallbladder inflammation ( Fig. 77-21 ). Altun and colleagues found that increased gallbladder wall enhancement and increased transient pericholecystic hepatic enhancement had the highest combination of sensitivity and specificity for the diagnosis and differentiation of acute and chronic cholecystitis. MR has been shown to be superior to ultrasound for detection of obstructing calculi in the gallbladder neck and the cystic duct ( Fig. 77-21C, D ). MRI may also be helpful to identify complications of acute cholecystitis. In the meta-analysis by Kiewiet and colleagues, diagnostic accuracy of MR for diagnosis of acute cholecystitis was found to be comparable to that of ultrasound, and it is suggested as a helpful imaging modality when ultrasound is technically limited. MRCP is more completely discussed in Chapter 75 .

Acute Acalculous Cholecystitis
Pathogenesis and Epidemiology
The reported incidence of acute cholecystitis in the absence of gallstones ranges from approximately 5% to 10%. In one large surgical series, acute acalculous cholecystitis (AAC) represented 14% of cases of acute cholecystitis and was responsible for 2% of all cholecystectomies. Bile stasis, gallbladder ischemia, cystic duct obstruction, and systemic infection are considered to be the most important factors in pathogenesis of AAC. Histologic features include gallbladder wall inflammation, with necrosis of blood vessels in the muscularis and serosa of the gallbladder. AAC occurs with increased incidence in patients who are critically ill or with prolonged illness, such as in the setting of trauma or after prolonged stay in the intensive care unit. Other risk factors include major cardiovascular disorders, cardiopulmonary bypass, diabetes, autoimmune disease, bacterial and fungal sepsis, hyperalimentation, and AIDS. Although it is much less common, the disease can occur de novo in the absence of these other risk factors in otherwise apparently healthy individuals.
AAC is a more fulminant form of acute cholecystitis with higher morbidity and mortality and rapid progression to gangrene and perforation. Mortality is reported to be as high as 65% ; therefore, early diagnosis is important. The diagnosis of AAC, however, often presents a challenge, particularly if there are no apparent risk factors. Clinical findings, such as fever and leukocytosis, are nonspecific. The diagnosis should be suspected in critically ill or injured patients who have fever or infection with no other apparent source.
Imaging
Ultrasound
The reported sensitivity of ultrasound in AAC varies widely from 36% to 92% ( Fig. 77-22 ). Gallstones are not present, and afflicted patients are often insensitive to pain because of altered mental status or medications, thus making the sonographic Murphy sign unreliable. These patients may also have hypoalbuminemia, congestive heart failure, and long-standing parenteral nutrition, all of which are associated with gallbladder wall thickening, distention, and sludge. There is a great degree of overlap in ultrasound findings of intensive care unit patients with AAC and those without. In one study evaluating the ultrasound findings in intensive care unit patients, the majority of patients had some abnormality of the gallbladder. Nevertheless, ultrasound has the advantage of being performed portably at the bedside and remains a reasonable first study for the diagnosis of AAC. Despite limitations, the American College of Radiology appropriateness criteria assign ultrasound the highest ranking for diagnosis of AAC.

Cholescintigraphy is also of limited value in the diagnosis of AAC, with a significant false-positive rate (nonvisualization of the gallbladder) of up to 40% in patients with hepatocellular dysfunction, prolonged fasting, or severe illness. A negative study is useful for excluding acalculous cholecystitis; however, a positive study must be interpreted with caution. The specificity can be improved to 88% with the use of morphine. A prospective study comparing ultrasound and morphine scintigraphy in the diagnosis of AAC found the sensitivity of ultrasound and morphine scintigraphy to be 50% and 67%, specificity 94% and 100%, positive predictive value 86% and 100%, negative predictive value 71% and 80%, and accuracy 75% and 86%, respectively. Combining the two modalities may lead to greater diagnostic accuracy.
Computed Tomography and Magnetic Resonance Imaging
CT or MRI may be helpful in the diagnosis of AAC in the patient who is stable enough to undergo imaging but should generally be performed as an adjunct to ultrasound. With the exception of the absence of gallstones, the CT and MRI findings of AAC are similar to those of calculous cholecystitis. The advantage of these modalities is that they may demonstrate pericholecystic inflammatory change and fluid and abnormalities of the gallbladder wall or adjacent hepatic parenchyma that may not be appreciated at sonography, allowing a more specific diagnosis ( Fig. 77-23 ). However, as at sonography, less specific findings that may be observed include gallbladder distention, sludge, and wall thickening. A study demonstrated, however, that a totally normal gallbladder at CT excluded the diagnosis of AAC.

Many times, these patients are too ill for additional cross-sectional imaging evaluation. If no other source of sepsis is discovered, it may be prudent to proceed with percutaneous cholecystostomy, which can be performed at the bedside. This has been shown to be a safe and effective procedure in the patient with acute cholecystitis who is not a surgical candidate. This can be helpful, for both diagnosis and treatment, in patients with sepsis of unknown cause and equivocal imaging findings of AAC. A study showed that percutaneous cholecystostomy was superior to gallbladder aspiration in terms of clinical effectiveness and was associated with the same complications.
Complications of Acute Cholecystitis
Gangrenous Cholecystitis
Gangrenous cholecystitis is a severe form of acute cholecystitis associated with vascular compromise and intramural hemorrhage, necrosis, and intramural abscess formation. This is usually caused by a stone impacting the cystic duct, with progressive distention of the gallbladder and ultimately ischemic necrosis of the wall. The incidence ranges from 2% to approximately 30% in various surgical series. The incidence is increased in men, patients of advanced age, and those with cardiovascular disease. Once it is diagnosed, treatment is generally emergency cholecystectomy to avoid life-threatening complications, such as perforation. There is a higher rate of conversion to open cholecystectomy than in uncomplicated acute cholecystitis.
At sonography, the features of gangrenous cholecystitis include heterogeneous, striated thickening and irregularity of the gallbladder wall and intraluminal membranes resulting from desquamation of the gallbladder mucosa ( Figs. 77-24 and 77-25 ). The irregular or asymmetric thickening of the gallbladder wall probably results from ulceration, hemorrhage, necrosis, or microabscess formation. In a series by Jeffrey and colleagues, these findings were present in 50% of patients. A striated appearance of the gallbladder wall was found in 40% of patients in the series by Teefey and associates. However, in a more recent analysis by this author, this finding was nonspecific for the presence of gangrenous cholecystitis and found in nongangrenous cholecystitis and other conditions that cause gallbladder wall edema, such as hepatitis. The presence of intraluminal membranes is considered a more specific finding (see Fig. 77-25 ). The sonographic Murphy sign may not be present because of associated denervation of the gallbladder wall. In a series by Simeone and colleagues, the sonographic Murphy sign was present in only 33% of patients with gangrenous cholecystitis. Additional findings include intramural abscess formation and pericholecystic fluid collection or abscess formation caused by perforation of the gallbladder.


CT findings of gangrenous cholecystitis parallel findings at sonography and include intraluminal membranes, intraluminal hemorrhage, irregularity or disruption of the gallbladder wall, and pericholecystic abscess ( Fig. 77-26 ). An additional finding detectable at CT is irregular or lack of gallbladder wall enhancement ( Fig. 77-27 ). A study by the author evaluated the sensitivity and specificity of CT for the diagnosis of gangrenous cholecystitis. CT was highly specific for identifying acute gangrenous cholecystitis (96%) but had low sensitivity (29.3%). The most specific findings at CT for the presence of gangrenous cholecystitis were gas in the gallbladder wall or lumen, intraluminal membranes, irregularity or absence of the gallbladder wall, pericholecystic abscess, and lack of gallbladder wall enhancement. In this study, the presence of pericholecystic fluid, degree of gallbladder distention in the short axis, and degree of mural thickening were also predictive of the severity of gallbladder inflammation. In a more recent study by Wu and associates, the presence of a perfusional defect of the gallbladder wall (discontinuity or decreased enhancement of the gallbladder wall) was associated with CT diagnosis of gangrenous cholecystitis with accuracy of 80%, sensitivity of 70.6%, specificity of 100%, and positive predictive value of 100%. Therefore, when CT is performed to evaluate for cholecystitis, contrast material should be administered intravenously if possible because this improves delineation of the wall and identification of lack of enhancement, intramural abscess, and focal disruption, which are important features of gangrenous cholecystitis.


Gallbladder carcinoma may be mimicked by the mural changes in acute cholecystitis, particularly gangrenous cholecystitis, and this is an important potential pitfall. If there is concern on the basis of ultrasound findings, CT may be of benefit in demonstrating an enhancing gallbladder mass and detecting direct invasion of the liver and presence of liver metastases. Liang and colleagues found that in the differentiation of acute cholecystitis and gallbladder carcinoma, features that favored carcinoma included focal gallbladder wall thickening, intraluminal mass, nondistended gallbladder with diffuse wall thickening, and enlarged regional lymph nodes.
Hemorrhagic Cholecystitis
Hemorrhagic cholecystitis is an uncommon complication of acute cholecystitis and usually occurs in the setting of cholelithiasis and gangrenous cholecystitis. Transmural inflammation causes mural necrosis and ulceration and results in hemorrhage into the gallbladder lumen. Atherosclerotic change in the gallbladder wall may be a predisposing factor. Blood clots in the lumen may become impacted in the cystic duct or common bile duct (CBD) or pass into the small bowel. The clinical presentation may be identical to uncomplicated acute cholecystitis with fever and right upper quadrant pain but may also include biliary colic, jaundice, hematemesis, and melena. Massive upper gastrointestinal bleeding and hemoperitoneum rarely occur. Prompt diagnosis is essential because of the associated high mortality rate.
At sonography, blood in the gallbladder lumen can be recognized as hyperechoic material that demonstrates greater echogenicity than sludge ( Fig. 77-28 ). This may form a dependent layer; however, clotted blood may appear as a clump or mass adherent to the gallbladder wall or heterogeneous echogenic material. An organized clot may simulate a polypoid, intraluminal mass. As the hemorrhage evolves, the clot may become more cystic in appearance. In addition to other findings of cholecystitis, CT shows increased density of bile (see Fig. 77-28B ). A fluid-fluid level may be observed with a high-attenuation dependent component simulating the hematocrit effect observed in acute hemorrhage ( Fig. 77-29A ). Other causes of high-density bile include biliary excretion of iodinated contrast material and milk of calcium; these do not generally appear as echogenic at sonography, nor is a fluid-fluid level observed. If there is associated perforation of the gallbladder, hemoperitoneum will also be observed ( Fig. 77-29B ). If there is active bleeding at the time of contrast-enhanced CT, this may appear as active extravasation of intravenous contrast material inside the gallbladder lumen. On MRI, blood products appear as high signal intensity within the gallbladder lumen on T1-weighted images and moderate to high heterogeneous signal intensity on T2-weighted images. The multiplanar capability of MRI may help differentiate intraluminal blood from hemorrhage in the wall. MR angiography may supplement conventional imaging if vascular disease is suspected.


Emphysematous Cholecystitis
Emphysematous cholecystitis is a rare life-threatening and rapidly progressive complication of acute cholecystitis. Cystic artery compromise is thought to promote the proliferation of gas-producing organisms in an anaerobic environment and penetration of gas into the gallbladder wall. The organisms most commonly isolated are Clostridium welchii and Escherichia coli . At pathologic examination, gallbladders with emphysematous cholecystitis have a higher incidence of endarteritis obliterans, supporting vascular insufficiency as a causative factor. This complication occurs with higher frequency in patients with diabetes (up to 50%) and male patients (up to 71%). Gallstones may be absent in up to one third of patients, and there is a high risk of gangrene and a perforation rate five times higher than in acute uncomplicated cholecystitis.
The mortality rate for emphysematous cholecystitis is 15% versus 4% in uncomplicated acute cholecystitis. Therefore, prompt diagnosis is critical. The clinical presentation is often indistinguishable from uncomplicated acute cholecystitis. In the diabetic patient, in particular, severe symptoms may be absent, and there needs to be a high clinical index of suspicion. Before cross-sectional imaging, emphysematous cholecystitis was diagnosed on the abdominal radiograph and classically described in three stages: stage 1, gas in the gallbladder lumen; stage 2, gas in the gallbladder wall; and stage 3, gas in the pericholecystic soft tissues. Ultrasound and CT are now considered more sensitive in the detection of smaller amounts of gas. Ultrasound findings vary by the amount and location of gas. A small amount of gas in the wall may appear as an echogenic focus with associated ring-down or comet-tail artifact. Larger amounts of gas and intraluminal gas may appear as a curvilinear arc of increased echogenicity with associated “dirty” posterior acoustic shadowing ( Fig. 77-30A ). In this setting, the gallbladder may be difficult to visualize owing to associated acoustic shadowing. On sonographic evaluation, it may be difficult to differentiate emphysematous cholecystitis from a contracted gallbladder with stones or a porcelain gallbladder with calcified wall.

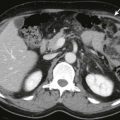
CT has a higher sensitivity than sonography for the identification of emphysematous cholecystitis and plays an important complementary role to ultrasound. If emphysematous cholecystitis is suspected at sonography or there is a high clinical index of suspicion and ultrasound findings are equivocal, CT should be performed. CT is also indicated when the gallbladder cannot be adequately visualized at sonography. CT findings include gas within the gallbladder wall or nondependent portion of the lumen ( Fig. 77-31 ). There may be extension into the pericholecystic soft tissues. CT may also demonstrate other complications, such as abscess formation and perforation ( Fig. 77-30C, D and Fig. 77-31B, C ). Free intraperitoneal gas indicates associated free gallbladder perforation and constitutes a surgical emergency.