Fig. 15.1
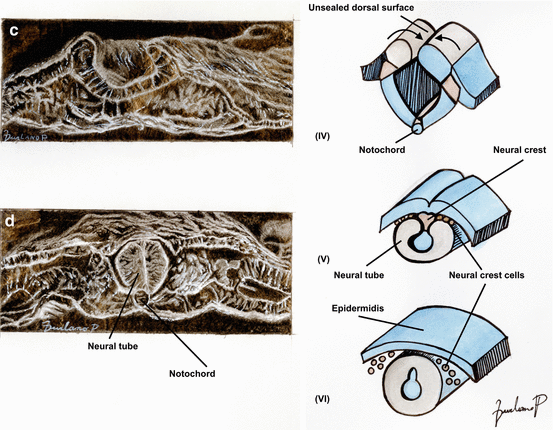
Artistic drawings show embryogenesis of the vertebral column: primary neurulation. (a) Shaping. Schematic transverse sections showing neuroectodermal tissues differentiate from the ectoderm and thicken into the neural plate (I); (b) Folding and elevation. The neural plate bends dorsally creating the U-shaped neural groove. Notochord is shown at the ventral part of the neural groove (II). The two ends eventually joining at the neural plate borders, which are now referred to as the neural crest (III); (c) Convergence. Bending of the neural plate with convergence of the neural folds up to the complete closure of the neural tube (IV); (d) Closure. The closure of the neural tube disconnects the neural crest from the epidermidis (V). Neural crest cells differentiate to form most of the peripheral nervous system and notochord degenerates (VI)
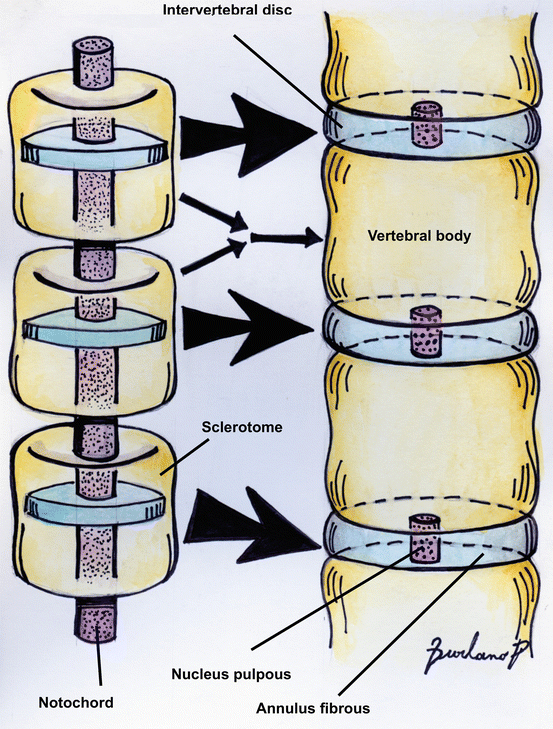
Fig. 15.2
Artistic drawing show notochord cells remain in the nucleus pulposus of the mature intervertebral disk. During resegmentation of the sclerotomes to form the vertebrae, each one splits into cranial and caudal segments, and cells remaining in the plane of division coalesce to form the annulus fibrous of the intervertebral disk
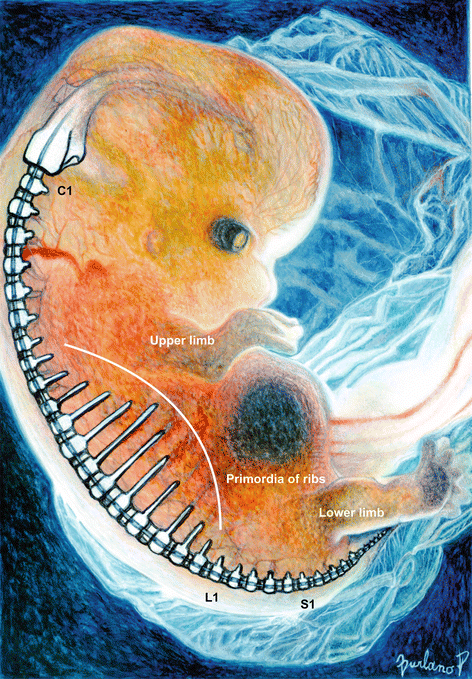
Fig. 15.3
Artistic drawing of a human embryo at Carnegie stage 20 show all of the vertebral segments have formed
15.3 Pathogenesis
The pathophysiology underlying this lethal disease is demonstrated to be complex. Starting from the histological characterization performed by Virchow in 1857 [30] up to the discovery of brachyury’s involvement, numerous progresses have been performed in the pathogenesis of this tumor. Examination of human embryos and fetus showed that notochordal cell nests topographically correspond and distribute to the sites of occurrence of chordoma and histological appearance of tumor cells led to hypothesize the notochordal origin [31, 32]. Molecular research has so far yielded significant findings on the mechanisms underlying the initiation and further progression of chordoma cells. The most compelling evidence of the notochordal hypothesis derived from researches focused on a transcription factor named “brachyury.” It is an important transcription factor in notochord development, but duplicated regions contained only the brachyury gene have been discovered in familial chordoma [33–36]. The remarkable overexpression revealed this transcription factor to be a crucial aspect of chordoma, although it is still unclear what role brachyury has in the pathogenesis [37]. Some other notochordal factors (Shh, Wnt, galectin-3, NCAM) seem to be relevant in notochord formation and in chordoma, as well as the other overexpression of cell cycle regulatory pathways and an activated receptor tyrosine kinase pathway [11]. The molecular biology process behind the initiation and progression of a chordoma needs to be revealed for a better understanding of the disease and to develop more effective therapies [38].
15.4 Epidemiology
Chordomas are classified on the basis of their location along the spine in sacrococcygeal, clival, cervical, thoracic, and lumbar (listed by the most frequent site) [1–3]. Recent studies reported an almost equal distribution in the clivus (32%), mobile spine (32.8%), and sacrum 29.2% [39]. Other epidemiological studies report that the sacrococcygeal area is the most common affect (40–50%) compared to clivus (35–40%) and vertebral bodies (20–40%) [40, 41]. Sacrococcygeal chordomas have very low incidence in patients below 40 years old and are more frequent in males (male to female ratio 2:1) [42–45].
15.5 Presentation and Diagnosis
Chordomas of the sacrum often present with non-specific symptoms which can delay diagnosis, such as localized deep pain or radiculopathies related to the spinal level at which they occur [46–49]. In advanced disease the tumors can present at the time of diagnosis as a slow-growing palpable mass associated with rectal or urinary dysfunction [46, 47, 50]. The average duration of symptoms is about 14 months (range, 4–24 months) [45]. Diagnosis is further complicated by the fact that lytic sacral lesions might be overlooked on plain radiographs of the pelvis, and CT or MRI studies are often not performed without a clinical suspicion of sacral tumor [45, 49]. Differential diagnosis for lumbosacral chordomas includes pilonidal sinus disease, deep abscesses, rectal sarcomas, and several retrorectal tumors (teratomas, extraperitoneal adenomucinosis, cystic lymphangiomas, neurogenic tumors, and cysts, developmental tailgut cysts, sacral myelomeningocele, rectal duplication) [51–53]. Most sacrococcygeal chordomas can protrude anteriorly into the pelvis, and rectal examination may be useful for clinical detection of sacral mass even if the tumor is limited by presacral fascia [54].
15.6 Imaging
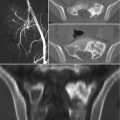
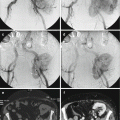
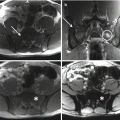
Chordomas are midline lesions and often appear radiographically as destructive lytic bone lesions. Unlike other primary tumor (osteosarcomas and chondrosarcomas) of the spine, chordomas locally invade the intervertebral disk space as they spread to adjacent vertebral bodies [49]. Computed tomography (CT) and magnetic resonance imaging (MRI) are the gold standard for diagnosis. The tumors are often associated with soft tissue mass (Fig. 15.4). Calcification and bony expansion are present in 30–70% of the cases and appear isointense or hypointense on T1-weighted MRI images and hyperintense on T2-weighted MRI images and enhance with gadolinium [55]. On bone scan, chordomas show reduced or normal uptake of radioisotope when juxtaposed to other bone tumors [56]. Careful preoperative assessment of imaging within the multidisciplinary team is essential in planning surgical approach and discussing strategy of treatment.
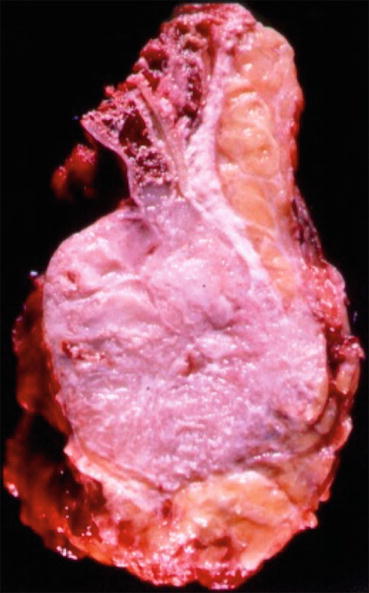

Fig. 15.4
Sagittal view of a specimen from a proximal sacral resection show the large involvement of soft tissue
15.7 Pathology
Chordomas were first described histologically by Virchow in 1857, when he identified the typical “physaliferous” tumor cell chordomas [30]. Physaliferous cells appear as large white cells with round nuclei and abundant vacuolated cytoplasm separated by fibrous septa into lobules [41, 42] and are typical of classic chordomas. Chordomas are classified as classical (or conventional), chondroid, or dedifferentiated [41]. Classic chordomas are pathologically identified by their typical cells and immunoreactivity for S-100, epithelial membrane antigen (MUC1), and cytokeratins [57, 58]. Brachyury staining is used to discriminate chordomas from other chondroid lesions [59]. Chondroid chordoma is a histological variant that account for 5–15% of all chordomas [41]. It shows histological features resembling chondrosarcoma, with hyaline cartilage associated with expression of chordoma markers [60, 61]. Dedifferentiated chordomas account for less than 10% of all chordomas and are characterized by sarcomatous areas with spindle cells such as fibrosarcoma, osteosarcoma, or rhabdomyosarcoma [62–66]. It is characterized by a fulminant clinical course resulting in metastases and/or death within 1 year of diagnosis in most of the cases [64–68].
15.8 Treatment
The most accredited treatment consists of surgical resection with wide margins, as no chemotherapy has been demonstrated to be effective against chordoma and conventional radiotherapy is only partially effective [5, 11, 69–74]. Complex pelvic anatomy coupled with the need of wide margins means that surgery is challenging to preserve essential neural function and avoid injury to visceral and vascular structures during resection. Frequently, margins are positive (marginal or contaminated) [11, 70, 71, 75, 76], and adjuvant treatment strategies must be considered.
15.8.1 Sacral Resection
In the 1970s, Stener and Gunterberg [77] first introduced the idea of wide en bloc surgical resection for the treatment of sacral tumors. Since then, en bloc resection has remained the mainstay of treatment of sacral chordoma worldwide, as reported by a large multicentric study based on the AOSpine Tumor Knowledge Forum Primary Spinal Tumor database [78]. En bloc sacrectomy is a highly demanding surgical procedure, consisting of a partial or total amputation of the sacrum. It is associated with significant soft tissue and skin defects, which may require reconstruction with myocutaneous flaps to reduce risk of wound infection and breakdown. However, it is attainable in more than 50% of sacral chordomas and offers the best long-term oncological outcomes when wide margins are obtained [69, 79].
The surgical approach is often planned according to tumor extension and level of resection. Usually all resections distal to S3 level could be approached posteriorly only [70, 80, 81], whereas proximal resections need a combined anterior-posterior approach [69–71, 78, 79, 82]. Advantages of the posterior approach are a single stage procedure and shorter operating time, whereas the combined approach enables the visceral organs to be dissected away from the tumor and protected during the osteotomy. Some exceptions have been reported in literature, using tools and innovative surgical techniques to perform proximal resections by a posterior approach only in selected cases [83, 84].
Unilateral or bilateral sacrifice of the nerve roots is necessary distal to resection, with corresponding functional damage [77, 82, 85–88]: motor and sensory deficits in the lower limbs are mainly related to the sacrifice of S1 and L5 nerve roots; sacrectomies that spare the S2 nerve root are associated with abnormal bladder and bowel function, even if better results can be expected if an S3 nerve root is also preserved; sexual dysfunction with relative saddle anesthesia is associated with bilateral S3 lesions, whereas unilateral sacrifice from S2 to S5 reduces but does not abolish urogenital and rectal functions. Numerous complications other than neurological deficits have been reported in literature, such as wound dehiscence, infection, iatrogenic visceral injury, hematoma, massive bleeding, liquoral fistula, flap necrosis, stress fractures, and other less frequently reported [70, 89]. Chen et al. [90] reported that albumin <3.0 g/dL, operating time (>6 h), and previous surgery were statistically significant risk factors for wound infection.
15.8.2 Radiotherapy
The use of radiotherapy (RT) as primary or adjuvant treatment for chordoma has been debated for several years and remains controversial. The majority of older publications that used conventional photon radiotherapy did not exceed 60 Gy, and investigators report poor local control in sacral chordomas [91, 92]. In fact, the problem of tolerance dose of the organs and tissues surrounding the sacrum results in the limitation of total RT dose that can safely be delivered to the tumor [93, 94]. Advances in radiation technology and treatment have led to more strategic targeting of neoplasms with higher doses of radiation. There is some consensus that the combination therapy of surgical resection and radiation therapy may be associated with higher rates of local control and overall survival [95].
Intensity-modulated radiation therapy (IMRT) and stereotactic delivery techniques can custom modulate each photon beam to conform to the tumor volume by minimizing the dose to surrounding tissues [92]. Some authors report the experience in sacral chordomas, finding that local control was significantly higher in patients treated with radiation dose delivered higher than 60 Gy (range 60–78 Gy) [96, 97].
Radiosurgery is a technique characterized by a very high dose of RT in a single fraction (or hypofractionated) thanks to the use of image-guided technology coupled with IMRT. The use of radiosurgery for chordoma has shown promising preliminary results for local control [98, 99].
Proton beam and heavy-ion particle radiation therapy are characterized by delivering a high-specific radiation dose to the tumor target volume and small dose to uninvolved normal tissue (low risk of radiation toxicity to neural tissue) [94, 100, 101]. Hadrons (high-dose protons or charged particles, including carbon ions, helium, or neon) provide biological and physical advantages in terms of their high relative biological effectiveness and reduced oxygen-enhancement ratio in the tumor region [102]. In fact, studies exploiting the use of hadron therapy in chordomas of the sacrococcygeal region show local control at 5 years of 60–70% [99, 103–105]. Compared with protons and photons, carbon ions have a relative higher biological effectiveness with a larger mean energy per unit length of their trajectory [91, 106]. Therefore, carbon-ion radiotherapy has been considered for treatment of unresectable chordoma [107]. Promising local control rate and better preservation of bladder-bowel function with the use of carbon-ion radiotherapy has been reported compared with surgery [107–109], whereas good results have also been reported as adjuvant treatment [110]. Unfortunately, the availability of hadron-based therapy is limited because of the associated construction and operational expenses [111, 112], even if the cost is expected to decrease rapidly [113].
Although there is limited literature comparing the effectiveness of newer radiation therapy modalities coupled with surgery, preliminary promising results have been reported with hadron therapy than with photon-based radiation [114–116]. However, at this time, single-fraction photon RT and proton-beam and carbon-ion RT with wide en bloc excision both are the accepted treatment standard in the management of chordomas at many quaternary-care cancer centers, showing higher local control rates than conventional IMRT [116]. One of the critiques of RT is that it can cause pathological fracture of residual sacral bone [69, 70, 117].
15.8.3 Chemotherapy and Medical Treatment
No conventional chemotherapy has proven to be effective in terms of overall survival and local control in patients with sacral chordoma. The advent of molecular targeted therapies and the discovery of molecular profiling of chordomas have offered some encouraging alternatives to conventional chemotherapy for the management of advanced disease. Chordomas overexpress platelet-derived growth factor receptor (PDGFR)B, PDGFRA, and KIT receptors, suggesting a role for imatinib therapy [118–120], a tyrosine kinase inhibitor (TKI) with specificity for the kinase domain of PDGFR and KIT receptors. Another TKI, sunitinib, has shown clinical efficacy [121], even if reports are limited by small patient numbers and short follow-up. Additional molecular pathways such as mTOR and MAPK signaling pathways seem to be involved, meaning a possible role of other targeting therapies such as mTOR inhibitors (everolimus, temsirolimus) [122, 123].
In a small series of patients with chordoma, strong expression of epidermal growth factor receptor (EGFR) and c-MET was described [124]. EGFR is a tyrosine kinase receptor implicated in cell proliferation through the binding of several ligands. This led to the report of a patient’s response to cetuximab and gefitinib [125]. Newer EGFR inhibitors, erlotinib and lapatinib, confirmed this efficacy [126, 127]. A recent analysis showed that activation of phosphorylated signal transducer and activator of transcription 3 (STAT3) is associated with poor prognosis [128]. The use of STAT3 inhibitors in chordoma cell lines in vitro showed strong inhibition of cell growth and proliferation [129]. Phase II studies are now ongoing, combining imatinib and everolimus or using lapatinib in HER2-positive advanced chordomas.
15.9 Oncologic Outcome
Several prognostic factors associated with poor survival have been reported: age [78, 130, 131], tumor size [46, 71, 72, 131–133], preoperative C-reactive protein >1.0 mg/dL [134], tumor site regarding the proximal extent of the tumor, local invasion into other tissues [131, 135], inadequate surgical margins [46, 69, 70–72, 75, 133, 136–138], content of extracellular matrix and high Ki-67 index [138], histological category such as dedifferentiated chordomas [41, 139], and local recurrence [46, 138, 140]. Patient survival seems to be less affected by distant metastasis than by local progression of the disease, underlining the role of local control to improve oncological outcomes.
Local recurrence (LR) after surgical treatment is common (43–85%) despite resection with adequate margins [71, 72]. Some authors suggested that infiltration of the musculature adjacent to the sacrum and/or involvement of the sacroiliac joints increases the tendency to local recurrence, even after apparently successful en bloc resection [135, 141, 142]. This hypothesis could also justify also the observation of similar recurrence rate between major resections of proximal part of the sacrum and small resections distal to S3 level [70, 71]. Therefore, in cases of infiltration of the sacroiliac joint, we should consider the tumor at an advanced stage with increased risk of satellite lesions which may promote disease recurrence. The factors associated with higher risk of local recurrence are: old age [82, 140], higher sacral localization [140], inadequate surgical margins [46, 69, 70, 78], and previous intralesional surgery [69–71, 78]. Adjuvant radiotherapy may improve oncological outcomes in patients with inadequate surgical margins or dedifferentiated disease, but optimal radiotherapeutic regimens with long-term survival have not been developed. In fact, despite combining en bloc resection with particle radiation therapy, frequent local recurrence remains a reality, and the 5- and 10-year overall survival rates are circa 65% and 35%, respectively [39, 143, 144].
Acknowledgements
We would like to thank Pierpaolo Furlano for the production of research drawings which formed the basis of the final drawings in this chapter.
Conflict-of-Interest Statement
No benefits have been or will be received from a commercial party related directly or indirectly to the subject matter of this article.
References
1.
Healey JH, Lane JM. Chordoma: a critical review of diagnosis and treatment. Orthop Clin North Am. 1989;20:417–26.PubMed
2.
Smoll NR, Gautschi OP, Radovanovic I, Schaller K, Weber DC. Incidence and relative survival of chordomas: the standardized mortality ratio and the impact of chordomas on a population. Cancer. 2013;119:2029–37.PubMed
3.
Fabbri N, Ruggieri P. Chordoma. In: Picci P, et al., editors. Atlas of musculoskeletal tumors and tumor like lesions. Switzerland: Springer; 2014. p. 233–8.
4.
Casali PG, Stacchiotti S, Sangalli C, Olmi P, Gronchi A. Chordoma. Curr Opin Oncol. 2007;19:367–70.PubMed
5.
Garofalo F, di Summa PG, Christoforidis D, Pracht M, Laudato P, Cherix S, Bouchaab H, Raffoul W, Demartines N, Matter M. Multidisciplinary approach of lumbo-sacral chordoma: from oncological treatment to reconstructive surgery. J Surg Oncol. 2015;112(5):544–54.PubMed
6.
Amichetti M, Cianchetti M, Amelio D, Enrici RM, Minniti G. Proton therapy in chordoma of the base of the skull: a systematic review. Neurosurg Rev. 2009;32:403–16.PubMed
7.
Staab A, Rutz HP, Ares C, et al. Spot-scanning-based proton therapy for extracranial chordoma. Int J Radiat Oncol Biol Phys. 2011;81:e489–96.PubMed
8.
Luschka H. Die Altersveränderungen der Zwischenwirbelknorpel. Virchows Arch A Pathol Anat Histol. 1856;9:311–27.
9.
Kyriakos M, Totty WG, Lenke LG. Giant vertebral notochordal rest: a lesion distinct from chordoma: discussion of an evolving concept. Am J Surg Pathol. 2003;27:396–406.PubMed
10.
Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13(2):e69–76.PubMed
11.
Yakkioui Y, van Overbeeke JJ, Santegoeds R, van Engeland M, Temel Y. Chordoma: the entity. Biochim Biophys Acta. 2014;1846(2):655–69.PubMed
12.
O’Rahilly R, Meyer DB. The timing and sequence of events in the development of the human vertebral column during the embryonic period proper. Anat Embryol. 1979;157(2):167–76.PubMed
13.
Schoenwolf GC, Larsen WJ. Third week: becomming trilaminar and establishing body axis, Larsen’s human embryology. Philadelphia: Elsevier/Churchill Livingstone; 2009.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree