© Springer International Publishing AG 2017
Pietro Ruggieri, Andrea Angelini, Daniel Vanel and Piero Picci (eds.)Tumors of the Sacrum10.1007/978-3-319-51202-0_2121. Tumors of the Sacrum: Diagnosis, Management, and Surgical Techniques
(1)
Section of Orthopaedic Oncology, Department of Orthopaedic Surgery, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, USA
21.1 Introduction
Latin for “sacred bone” and so named for its recurrent role in ancient Greek and Egyptian mythology [1], the os sacrum remains the subject of much discourse and scholarship in modern-day musculoskeletal oncology. While certain sacral tumors, including hematologic malignancies, giant cell tumors, and the majority of metastatic lesions, may be managed medically, chordomas, chondrosarcomas, and other primary malignancies typically warrant resection. The surgical treatment of these tumors demands careful attention to the complex interplay of anatomic, biomechanical, and oncologic factors. However, with meticulous preoperative planning and input from a specialized multidisciplinary team, good functional and oncologic results can be obtained.
21.2 Anatomy
The surgical management of sacral tumors requires a detailed understanding of the bony, ligamentous, vascular, and nervous anatomy of the pelvis [2].
Bony anatomy: A single bone formed by the fusion of five vertebrae, the sacrum articulates laterally with the ileum via paired L-shaped facets. Inferior articulation (or fusion) with the coccyx involves the two horn-like coccygeal cornua and their sacral counterparts. Four pairs of anterior and four pairs of posterior foramina carry the anterior and posterior rami, respectively, of the S1–4 nerve roots, as they emerge from the sacral canal. The termination of the sacral canal, which itself is the caudal continuation of the vertebral canal, is the sacral hiatus.
Ligamentous and articular anatomy: The articulation between L5 and S1 involves the two zygapophyseal joints and the intervertebral disk. The L5/S1 disk is wedge-shaped, thicker anteriorly than posteriorly, contributing to the lordosis at this level. Stout iliolumbar and lumbosacral ligaments, extending from the transverse processes of L5 to the ilium and sacrum, reinforce the lumbosacral junction. The synovial sacroiliac joints, prone to fibrosis and fusion with aging, are likewise stabilized by thick anterior and posterior ligaments. Finally, the sacrospinous and sacrotuberous ligaments serve to stabilize the bony pelvis, reinforce the lateral pelvic walls, and define the greater and lesser sciatic foramina.
Muscular anatomy: Relevant muscular anatomy about the sacrum includes the paired piriformis and coccygeus muscles, as well as the anococcygeal ligament. The piriformis originates on the anterior surface of the sacrum and exits the pelvis through the greater sciatic foramen, en route to its tendinous insertion on the greater trochanter. The piriformis serves as an important landmark within the greater sciatic foramen; contents of the suprapiriform foramen include the superior gluteal nerve and vessels, while structures exiting inferiorly include the inferior gluteal vessels, sciatic nerve, pudendal nerve, internal pudendal vessels, posterior femoral cutaneous nerve, and nerves to the obturator internus and quadratus femoris.
Contents of the lesser sciatic foramen, separated from its superior counterpart by the sacrospinous ligament, include the tendon of the obturator internus as it exits the pelvis and the pudendal nerve and internal pudendal vessels as they reenter the pelvis. The coccygeus, which originates on the inner surface of the sacrospinous ligament, inserts on the lateral borders of the sacrum and coccyx. The anococcygeal ligament is formed at the midline raphe of the left and right levator ani musculature, which constitutes the pelvic floor and helps to maintain anal and vaginal closure. Posteriorly, the anococcygeal ligament inserts on the coccyx.
Peritoneal anatomy: The sacrum is a retroperitoneal structure. It should be noted that the rectum is retroperitoneal as well; the upper two-thirds of this structure is draped anteriorly by peritoneum, while the lower one-third is completely uncovered by peritoneum.
Vascular anatomy: The internal iliac vascular system is relevant to surgery of the sacrum and pelvis. The paired internal iliac (hypogastric) arteries typically branch from the common iliac arteries at the level of L5/S1, anteromedial to the SI joint. At the superior border of the greater sciatic foramen, the internal iliac artery divides into anterior and posterior trunks, which each subsequently give rise to multiple named vessels. The posterior trunk supplies the posterior pelvic wall and gluteal region; branches include the iliolumbar artery, which ascends superiorly out of the posterior pelvis and gives off a spinal branch that passes through the L5/S1 intervertebral foramen; the lateral sacral artery, which gives multiple branches that pass into each anterior sacral foramina; and the superior gluteal artery, which exits the pelvis through the suprapiriform greater sciatic foramen and supplies the abductor musculature.
Relevant branches of the anterior trunk of the internal iliac include the internal pudendal artery, which runs through Alcock’s canal with the pudendal nerve and supplies the external genitalia; the obturator artery, which exits the pelvis through the obturator foramen into the adductor compartment of the thigh; the inferior gluteal artery, which exits the pelvis through the infrapiriform greater sciatic foramen and supplies the gluteus maximus and piriformis; and multiple branches to the pelvic viscera. The median sacral artery, an unpaired, midline vessel, branches off the abdominal aorta just proximal to its bifurcation and travels down the anterior surface of the sacrum and coccyx.
Nervous anatomy: The sacral and coccygeal plexuses, formed by the anterior rami of S1-Co with contributions from L4 to L5, carry primarily somatosensory fibers, with sympathetic and parasympathetic components as well. As noted above, four paired anterior and four paired posterior foramina transmit the anterior and posterior rami of the S1–4 nerve roots. Each anterior ramus, except at the S4 level, in turn divides into ventral and dorsal divisions.
The sacral plexus gives rise to multiple somatic nerves, including the sciatic (L4–S2), superior and inferior gluteal, and pudendal nerves, as well as smaller motor branches to the quadratus femoris, gemelli, obturator internus, levator ani, and coccygeus muscles, and two sensory nerves (the posterior femoral cutaneous and perforating cutaneous). The pudendal nerve is of particular importance to surgery of the sacrum and pelvis. Arising from the ventral divisions of the anterior rami of S2–S4, the pudendal nerve exits the pelvis through the infrapiriform greater sciatic foramen, passes dorsal to the sacrospinous ligament, and reenters the pelvis through the lesser sciatic foramen. As it does so, it courses along the lateral wall of the ischioanal fossa within Alcock’s canal (pudendal canal), inferior to the pelvic floor and accompanied by the internal pudendal vessels. The pudendal nerve innervates the levator ani as well as the skeletal muscle of the external anal and urethral sphincters and provides sensory innervation to much of the perineum and penis or clitoris. Compression or injury to the pudendal nerve or its sacral nerve roots can result in bowel, bladder, and sexual dysfunction.
In addition to its somatic nervous functions, the sacral plexus provides parasympathetic visceral innervation through its contributions to the inferior hypogastric plexus of the pelvis. Functions of parasympathetic pelvic innervation include vasodilation of the erectile tissue of the penis or clitoris, stimulation of bladder contraction during micturition, and modulation of activity of the descending colon and rectum. This parasympathetic outflow is carried by the pelvic splanchnic nerves, which originate primarily from the S2–S4 roots. The pelvic splanchnic nerves join fibers from the superior hypogastric plexus, which descends along the posterior abdominal wall and carries both sympathetic and parasympathetic fibers, to form the inferior hypogastric plexus.
Sympathetic functions of the inferior hypogastric plexus include innervation of smooth muscle within the pelvic vasculature, internal anal and urethral sphincters, and reproductive tract (i.e., critical for the processes of ejaculation). It should be noted that sympathetic fibers within the inferior hypogastric plexus are supplied by the roots of T10–L2, but not by sacral nerve roots. Finally, the somatic coccygeal plexus, with contributions from S4, S5, and Co, gives rise to the anococcygeal nerve, which contributes to motor and sensory innervation of the perineum.
21.3 Applied Surgical Anatomy: Biomechanical and Neurologic Considerations
The anatomic features of the pelvis inform the biomechanical and functional considerations relevant to planning for sacral resections. Partial transverse sacrectomies have been classified with respect to nerve root anatomy, wherein preservation of S3, S2, and S1 corresponds to low, middle, and high sacrectomies, respectively [3]. Level of resection is of critical importance not only with respect to margin status but also with respect to preservation of mechanical stability of the pelvis and maintenance of bowel, bladder, and sexual function.
21.3.1 Biomechanical Considerations
Spinopelvic fixation is typically performed after total sacrectomy; in the absence of reconstruction, as elaborated by one author, the resultant “flail axial skeleton precludes the ability to ambulate” [4]. On the other hand, low partial sacrectomy does not warrant reconstruction. However, the indications for reconstruction after high or middle partial sacrectomy are less clear. Early biomechanical work performed by Gutenberg [5] found that resection through (or just cephalad to) the S1 foramina weakened the pelvic ring by 50%, while resection between the S1 and S2 foramina resulted in only 30% weakening. A more recent cadaveric study, which purported to model physiologic loading more accurately, found that pelvises with sacral resections just caudal to the S1 foramina could withstand forces associated with postoperative mobilization, while those with resections just cephalad to the S1 foramina could not [6]. These authors highlighted the importance of (at least partial) preservation of the sacroiliac joint and noted that bone cuts just cephalad vs. just caudal to the S1 foramina are associated with preservation of 75% vs. 84%, respectively, of the sacroiliac joint—perhaps signifying a biomechanically significant “cutoff” point within that range. Clinical outcome data have largely confirmed these findings. One review found that three of nine sacrectomies involving the S1 body failed via fracture, ultimately requiring reconstruction [7]; another series from our institution, in which high-dose adjuvant radiation was utilized, reported a 76% rate of postoperative sacral insufficiency following high sacrectomy, as compared with 0% after low sacrectomy [8]. Not all authors advocate for reconstruction after total or high sacrectomy: Ruggieri et al. [9] have suggested that muscle and scar tissue may form a “biologic sling” between the unreconstructed pelvis and lower lumbar spine, which may migrate inferiorly toward the ilia. However, it is our preference to perform spinopelvic reconstruction for any sacrectomy cephalad to the S3 foramina when adjuvant radiotherapy (and the attendant risk of fracture) is utilized.
21.3.2 Neurologic Considerations
The S3 nerve roots have traditionally been thought to play a critical role in supplying normal bowel, bladder, and, to a lesser extent, sexual function. Sacrectomy with nerve root sacrifice cephalad to S3 may result in loss of normal bowel and bladder function in many, if not all, patients [3, 5, 7, 10, 11]. Specifically, S2 may allow for weak internal and external anal sphincter activity, but not for discrimination between different rectal contents or sensation of rectal distention, nor for maintenance of the micturition reflex. A review of 53 sacrectomies performed at the Mayo Clinic found that preservation of bilateral S3 nerve roots ensured maintenance of normal bowel and bladder function in 100% and 69% of patients, respectively; unilateral S3 preservation ensured maintenance of function in approximately two-thirds of patients. All patients with sacrifice of bilateral S2 roots had abnormal bowel and bladder function, and a minority of patients enjoyed normal bowel and bladder function with bilateral S3 sacrifice [7]. However, a more recent case series [10] found slightly improved outcomes with S3-sacrificing sacrectomies: normal bowel and bladder function in 63% and 71%, respectively. Preservation of the S2 nerve roots—and perhaps even S1 nerve roots alone—might be sufficient for maintenance of sexual function [5, 12–14]. Unilateral S1–5 resection, with preservation of contralateral nerve roots, has minimal impact on bowel, bladder, or sexual function [3, 5, 7].
Investigations of patient-reported outcomes at our center, utilizing PROMIS and other subjective patient response questionnaires, have confirmed the negative impact of more proximal resections, while demonstrating that postoperative bowel and bladder deficits exist along a spectrum. Phukan et al. [15], in a review of survey data from 33 patients, reported a stepwise decrease in voiding, continence, and defecation scores in patients with S4, S3, S2, and S1 partial sacrectomies, respectively. A similar downtrend in defecation scores among patients with S4, S3, and S2 partial sacrectomies was observed in a study of questionnaire data in 74 sacrectomy patients at our institution and two others [14]. Taken together, these results argue against a binary model of sacral nerve root contribution to bowel and bladder function: preservation of the S3 nerve roots, while ideal, may be neither sufficient nor necessary in all cases for maintenance of normal voiding and defecation.
Evaluation of PROMIS questionnaire data also demonstrated that high partial and total sacrectomies were consistently associated with chronic pain and significantly lower physical and mental health scores in patients with resections cephalad to S3 [14, 15]. As with bowel, bladder, and sexual function, therefore, chronic pain must be addressed in preoperative patient discussions as a known risk of high sacrectomy. Notably, however, quality of life and functional scores following low sacrectomy were in fact equivalent or superior to normative (general population) PROMIS data [15].
It should also be noted that the level of bony resection does not necessarily correlate with the extent of sacral root sacrifice: intraoperative margin considerations might require sacrifice of more cephalad nerve roots, or conversely, tumor location might allow for the sparing of nerve roots contralateral or caudal to the bone cut. Neurologic dysfunction might also result from disruption (caused by tumor or surgery) of the pudendal nerves or the inferior hypogastric plexus, even if the sacral nerve roots are preserved intraoperatively. Indeed, it has been demonstrated that the greatest predictor of postoperative bowel and bladder outcome is preoperative function [10]. Finally, sacral nerve root status is only one of a number of factors that may contribute to postoperative function. High partial sacrectomies may necessitate lumbo-pelvic fixation, which can be associated with sciatic nerve dysfunction or pain-generating hardware failure.
21.4 Clinical Management of Sacral Tumors
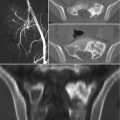
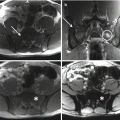
Sacral tumors may present with pain, perineal sensory changes, and sexual, bowel, and bladder dysfunction, the latter of which may be the result of either nervous or direct visceral compression. Additionally, sacral tumors may be quite large before symptoms arise. As for evaluation of any bone tumor, initial work-up should consist of history, physical examination, and imaging studies, including plain radiographs, computed tomography (CT) and magnetic resonance imaging (MRI) of the sacrum and pelvis, and CT of the chest and bone scan for staging. Complete imaging of the mobile spine should be performed as well; additional lesions may be present in 17% of patients with sacral chordomas [16].
Tissue is necessary for histologic analysis and should preferably be obtained through image-guided needle biopsy. Open incisional biopsy of chordomas, especially when performed outside of the ultimate treating center, is associated with a higher risk of local recurrence, metastasis, and tumor-related death [3, 17, 18]. Analysis of sacral mass biopsy tissue should include immunohistochemical staining for cytokeratins, EMA, vimentin, and brachyury, which stain strongly in chordoma tissue, as well as for Ki-67, which is associated with a poor prognosis when present with a high degree of proliferative activity in chordoma tissue [17]. Genetic analysis is also emerging as a component of the clinical work-up, as knowledge increases regarding the link between chordoma and brachyury, a notochordal “master regulator” transcription factor [19]. Genetic duplication of the brachyury locus is typical in familial chordomas, and a specific single-nucleotide variant in the gene has been identified in 86–94% of chordoma patients and associated with a sixfold increase in risk of chordoma development [19–21].
Tumor characteristics dictate surgical management, including extent of resection and use of adjuvants. Chordomas are the most common primary sacral malignancy; sacral chondrosarcomas, osteosarcomas, and Ewing sarcomas are seen as well. Benign primary tumors include giant cell tumors, aneurysmal bone cysts, and osteoid osteomas/osteoblastomas. Metastatic disease, multiple myeloma, and lymphomas are commonly encountered as well. Teratomas are the most common sacral tumors in children [22].
21.4.1 Management of Chordomas
Deriving from notochordal tissue, chordomas represent 1–4% of all primary malignant bone tumors, with an incidence of 0.08 per 100,000 [23, 24]. While chordomas have traditionally been thought to occur more commonly in the sacrum (50%) than in the skull base (35%) or the mobile spine (15%) [24], more recent data from the population-based SEER registry suggest that these tumors may occur with equal frequency in the skull base, mobile spine, and sacrum [23]. Reported overall 5-year survival in patients treated with sacral chordomas has ranged from 68% to 97% in the literature [9, 17, 23, 25–27], with median survival between 6 and 7 years in three large series [20, 23, 28].
Intralesional resection (or resection with inadequate margins) is associated with a higher rate of local recurrence—up to 83%—and, in some series, decreased survival [9, 11, 17, 20, 24–26, 28, 29]. Fuchs et al. [25], for instance, reported on 52 primary sacral chordomas treated surgically at the Mayo Clinic and noted that all 21 patients who underwent resection with wide margins were alive at 8-year follow-up, while two-thirds of patients with inadequate margins experienced local recurrence and two-thirds of those with local recurrence died within the study period. Bergh et al. [17] reported that local recurrence was associated with a 23-fold increased risk of metastases and a 21-fold increased risk of tumor-related death in a cohort of 39 chordoma patients, while Young et al. [30], reviewing 219 chordoma patients, reported a more modest 2.5-fold increased metastatic risk in cases of local recurrence, with an overall metastatic rate of 18%. Wide, en bloc resection is therefore recommended in the management of chordomas.
It has been suggested that tumor invasion into the piriformis or gluteus maximus musculature, or involvement of the sacroiliac joints, is an independent predictor of local recurrence, regardless of margin status at time of resection [29]. Indeed, local recurrences may tend to occur most frequently in the posterior musculature, and wider margins may be required posteriorly as compared with anteriorly, where the presacral fascia may pose a barrier to tumor spread [27, 29].
High-dose (70.2 Gy) proton/photon-beam radiotherapy is now standard in the management of chordomas and other spinal malignancies at our institution. For sacral tumors, preoperative radiation of either 19.8 or 50.4 Gy is administered, with the remainder administered postoperatively; in cases of positive surgical margins, localized boosts are utilized as well. A phase II clinical trial performed at our institution, evaluating 50 patients undergoing surgical resection of spinal chordomas and sarcomas (predominantly sacral), demonstrated that high-dose radiotherapy in addition to surgical resection was associated with a 74% rate of local control at 8-year follow-up [31, 32]. Notably, local control for primary tumors (94% at 5 years and 85% at 8 years) was far superior to that for locally recurrent tumors (~50% re-recurrence rate).
Chordomas, in particular, appear to benefit from high-dose radiation. A retrospective review of 127 spinal chordomas (including recurrent tumors) treated at our institution demonstrated 5-year overall survival and local control of 81% and 62%, respectively [26]. This study found further improvement in local control when surgical resection of primary chordoma was accompanied by neoadjuvant and adjuvant radiation, as opposed to adjuvant radiation alone: 85 vs. 56% at 5 years. Most strikingly, among the 28 patients with primary tumors in this series who underwent en bloc resection and received both neoadjuvant and adjuvant radiation, no cases of recurrence were observed.
Even in cases of margin-positive primary resection, good results may be salvaged: the use of adjuvant high-dose radiation achieved local control at 8.8 years in 10 of 11 patients with primary sacral chordomas resected with positive surgical margins (but in 0 of 5 patients with recurrent disease) treated at our institution [33]. Taken together, these results highlight not only the excellent outcomes achieved with wide resection plus neo- and adjuvant radiation, but also the critical importance of initiation of aggressive treatment at first presentation.
Additionally, in patients for whom surgical resection is not feasible due to risk of intraoperative nervous injury (i.e., high sacral tumors) or medical comorbidities, definitive management with high-dose radiation is reasonable. In a review of 24 spinal chordomas, of which 19 were located in the sacrum, treated at our institution with proton or photon radiotherapy alone, local control rates were 90.4% and 79.8% at 3 and 5 years, respectively. All surviving patients maintained ambulatory status [34]. A follow-up study noted ongoing tumor volumetric reduction up to 5 years after definitive radiation treatment [35]. Definitive carbon ion radiotherapy, which is characterized by a higher biological effectiveness than proton therapy and may be particularly effective against hypoxic tumors [36], may also be utilized. Five-year local control rates of 77–88% in patients with proximal, unresectable sacral chordomas have been published by a group in Japan [37, 38], while a group in Germany has reported 53% local control and 100% survival among a mixed cohort of primary and recurrent tumors treated with carbon ion radiotherapy [39].
However, high-dose radiation to the sacrum can be associated with significant adverse effects. Specifically, delivery of greater than 77 Gy, which is the standard dose at our institution in cases of definitive treatment with radiation alone, has been associated with higher rates of neuropathy and erectile dysfunction as compared with patients receiving ~70 Gy or less [26, 31, 32, 33, 38]. Sacral insufficiency fractures have been noted to occur in roughly half of all patients with sacral chordomas treated with definitive high-dose radiation [34, 35], and in over three-quarters of patients undergoing high sacrectomy with radiation [8]. For this reason, we avoid radiation doses greater than ~70 Gy in patients undergoing surgical resection.
Though the role of medical therapy in the clinical management of chordomas is currently limited, potential molecular targets for future drug development include the mechanistic target of rapamycin (mTOR) signaling pathway [40], as well as vascular endothelial growth factor (VEGF)-mediated angiogenesis and other receptor tyrosine kinase pathways [36].
21.4.2 Management of Other Sacral Malignancies
Chondrosarcoma of the sacrum represents approximately 20% of spinal chondrosarcomas and 5% of all chondrosarcomas [41], and is the second most commonly resected primary sacral tumor after chordoma [3, 32, 42]. As is the case with other sacral malignancies, en bloc resection with negative margins is likely associated with decreased rates of local recurrence and improved disease-free survival [41, 43, 44]. Chondrosarcomas, like chordomas, are treated with high-dose neo- and adjuvant radiation and wide surgical resection at our institution [32]. Spinal osteosarcoma is rare, but has been reported to occur in the sacrum in 31–68% of cases [45, 46]. At our institution, treatment includes en bloc resection, high-dose radiotherapy, and neo- and adjuvant chemotherapy [46], though outcome is poor and prognosis is worse for osteosarcoma of the sacrum as compared with that of the mobile spine [45].
Locally recurrent rectal cancer may be treated with aggressive re-resection, to include partial sacrectomy in cases of cortical invasion or tumor adherence to the bone. Overall mean survival of 22–40 months has been reported following re-resection with sacrectomy, with improved outcomes in cases of negative margins [47–49].
21.4.3 Management of Giant Cell Tumors
Traditionally, giant cell tumors (GCTs) of the sacrum have been treated with intralesional curettage, but high rates of recurrence—in up to one-third to one-half of cases—have been reported [50–52]. The authors of a review of a pooled cohort of 166 patients with sacral GCTs, therefore, recommended wide surgical resection for lower sacral lesions and for recurrent proximal sacral lesions [50]. Notably, this study reported a 23% disease-related mortality, of which approximately one-third was related to treatment complications, at 8-year follow-up. Radiation may be utilized in cases of large or challenging sacral GCTs, but is associated with high recurrence rates when used alone or as an adjuvant following curettage [50]. Radiation-related malignant transformation is also a concern [50, 52, 53]. Arterial embolization may represent a more successful nonoperative modality, and good results with respect to symptomatic improvement and low rates of recurrence have been reported with the use of serial arterial embolizations (typically performed every 4–6 weeks) as monotherapy for sacral GCTs [54–56]. Additionally, embolization may be performed concurrently with local intraarterial injection of cisplatin [56] or may be employed as a preoperative adjunct to limit surgical bleeding [51].
The development of denosumab has significantly increased the role of medical management in GCT treatment. A monoclonal antibody against receptor activator of nuclear factor-kappa B (RANK), denosumab inhibits the activation of osteoclast-like multinucleated cells, which express RANK ligand, thereby decoupling the osteoclastic pathway. Prolonged treatment results in marked depletion of giant cells and decreased cellularity overall on histologic analysis [57]. Phase II trials have demonstrated objective tumor response in 72–88% of patients, with significant improvements in pain reports as well [58, 59]. Among the subgroup of patients with tumors deemed operable but with high risk of morbidity, Chawla et al. [58] found that nearly three-quarters were able to avoid surgery altogether after denosumab treatment and that 62% of those who did ultimately undergo resection were managed with a smaller procedure than initially planned. At our institution, a 3–6 month course of denosumab “pretreatment” is typically pursued prior to surgical resection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree