Study
Patients (number)
Age/Gender
Primary tumor
Metastasis level
Neoadjuvant treatments
Procedure
Survival
Ozdemir et al. [59]
65; 34 sacral metastases
21M/25F; mean age, 49 years (range, 12–83 years)
Breast (9), MM (7), prostate (4), lung (3), colon (3), leiomyosarcoma (2), lymphoma (2), ovary (1), RCC (1), thyroid (1), larynx (1)
N/A
N/A
Resection or curettage and PMMA
36 months (30–120)
Turgut et al. [64]
1
63M
Merkel cell cancer
L5–S1
None
L5 laminectomy and subtotal resection
2 months
Lee et al. [65]
1
51F
Meningioma
Left sacrum and ilium
None
2-stage excision
N/A
Kollender et al. [9]
14; 5 sacral metastases
7M/7F; mean age, 42 years (range, 14–74 years)
RCC (2), MC (2), colon (1)
Mid sacrum (2), left sacrum (2), entire sacrum (1)
All RT; embolization (2)
Decompression and cryosurgery
6–36 months
Nader et al. [60]
19
N/A
RCC (13), breast (2), melanoma (1), MM (1), colon (1), liposarcoma (1)
S1 (18), S2 (13), S3 (3), S4 (2), L/S (4), SIJ (4), other spine (10), extraspinal (13)
N/A
Laminectomy (18), vertebrectomy (13), instrumentation (12)
22 months (5–38)
Menegaz et al. [66]
1
45F
Chorioncarcinoma
L2–S1
None
CMT and RT
7 months
Uemura et al. [67]
1
76M
HCC
Left and right sacrum
Embolization and RT
Sacroplasty
72 days
Gerszten et al. [68]
393; 500 lesions; 103 sacral metastases
251M/142F; mean age, 56 years (range, 18–85 years)
RCC (93), breast (83), lung (80), colon (32), sarcoma (26), prostate (24), MM (18), unknown (14), SCC (12), thyroid (11), other (69)
Sacral (103)
RT (344)
Radiosurgery
21 months (3–53)
Akasu et al. [69]
44
35M/9F; mean age, 55 years (range, 32–73 years)
Recurrent rectal cancer
Pelvic (12), solitary pelvic (24), distant (8)
RT (13)
Sacral resection
2.3 years (1–16)
Fujibayashi et al. [70]
5
4M/1F; mean age, 47 years (range, 29–77 years)
Lung (2), renal (2), paraganglioma (1)
LS joint, sacrum
N/A
Instrumentation
28 months (3–72)
Kakutani et al. [71]
1
52M
Melanoma
Left S1–4 and ilium
None
CMT and RT
9 months
Zhang et al. [72]
2
1M/1F; age, 62 and 38 years
Lymphoma, lung
S1 and T12, L1–3; S1
CMT and RT
S1 percutaneous sacroplasty
N/A
Albareda et al. [73]
1
62F
Endometrial
S4
None
En bloc resection
26 months
Toro et al. [74]
1
65M
HCC
Right S1–5, left S1–2
None
Sacroplasty
N/A
Dozois et al. [75]
9
7M/2F; mean age, 63 years (range, 38–78 years)
Recurrent rectal
S2 (6), S1 (2), L/S (1)
RT (7), CMT (1)
High sacrectomy
30% at 5 years
Nebreda et al. [76]
1
48F
Small-cell lung cancer
Left S1–2 and SIJ
CMT and SRS
PMMA
40 days
Feiz-Erfan et al. [10]
25
21M/4F; mean age, 57 years (range, 25–71 years)
RCC (15), prostate (3), other (7)
Solitary sacrum (12), multiple sites (13)
RT (11), CMT (9), embolization (12), other (5)
En bloc resection and instrumentation
11 months (5.4–16.6)
Moussazadeh et al. [77]
25; 31 procedures
10M/15F, mean age, 65 years, (range, 32–84 years)
Sacral mets (15), w/previous RT for pelvic cancer (4), chordomas (3), osteoporotic (5), other (4)
N/A
RT (22)
Sacroplasty w/PMMA
15 died at 4.3 months
Radiation therapy is an integral part of treatment for patients with metastatic bone disease, both in the upfront and adjuvant setting. Radiation therapy is frequently chosen as a first-line initial therapy for radiosensitive sacral metastases, in patients without evidence of spinal instability or acute neurological deterioration, where pain reduction and neurological improvement are feasible [78, 79]. It must be taken into account that radiosensitivity varies among primary cancer types. In general, prostate and lymphoid tumors are radiosensitive, and breast cancer is 70% sensitive and 30% resistant, whereas gastrointestinal tumors, renal cell carcinomas, and melanomas are radioresistant [3]. Patients are unlikely to experience complete relief immediately, but should expect pain relief within 4–8 weeks following treatment. An emerging form of radiation therapy, which allows more precise radiation delivery and high-dose hypofractionation, is spinal stereotactic radiosurgery (SRS). SRS allows administering a tumoricidal radiation dose even for radioresistant tumors, with minimal exposure of the surrounding normal tissues. Current commercial spinal SRS systems include the CyberKnife® (Accuray Incorporated, Sunnyvale, California) and Novalis® (BrainLAB, Heinstetten, Germany) [3]. Unlike conventional radiation therapy in which a full dose is delivered to both the vertebral body and the spinal cord or cauda equina, SRS can deliver a high-dose single fraction to the target tissue while sparing most of the adjacent neural elements, thus significantly reducing the possibility of radiation-induced myelopathy or injury to the nerve roots. Other major benefits of SRS include the relatively short treatment time, which can be in an outpatient setting and the minimal or complete absence of side effects.
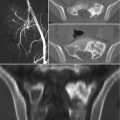
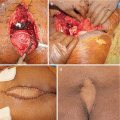
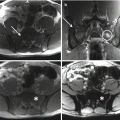
The role of surgery in metastatic disease of the sacrum is not clearly defined. Indications for surgical intervention include progressive neurological dysfunction or persistent pain that is unresponsive to radiation therapy, the need for a diagnostic biopsy, and pathological sacro-pelvic instability [3, 59]. If nonsurgical measures fail and the patient is suffering from symptomatic sacral disease, surgery can be a reasonable therapeutic option [10]. Metastatic disease in the sacrum generally does not cause mechanical instability, as this segment is rigidly fixed to the pelvis by the bilateral sacroiliac joints and their accompanying ligaments. In cases of massive tumor destruction of the S1 body and/or L5–S1 junction, fixation from the low lumbar spine into the sacrum and pelvis may be required to restore spinopelvic continuity. If instability occurs in the sacrum, reconstruction often includes low lumbar pedicle-based instrumentation systems along with sacral pedicle instrumentation and iliac bolts, with or without additional sacroiliac screws. A posterior midline sacral laminectomy may be necessary in case of sacral nerve root compression. In any case, care must be taken in the decision-making process, as sacral resections are challenging operations with a high incidence of potential complications, especially in patients with an already limited life expectancy [80]. On the other hand, resective surgery in carefully selected patients with sacral metastases may result in a palliative benefit. Removal of secondary lesions of renal cell carcinoma tends to be associated with an increase in overall survival. A minimal disease burden (single-site metastasis) may signify that there is a greater chance for surgery to achieve definitive local control [10].
Sacroplasty is a therapeutic option more extensively described in the degenerative/osteoporotic literature, now gaining favor in cases of metastatic disease without instability or neurologic compromise [81–83]. The technique is similar to vertebroplasty, involving cement augmentation of a metastatic lesion under CT guidance. Potential complications include hemorrhage, infection, dural tears with cerebrospinal fluid leak, direct injury of nerve roots or the lumbosacral plexus, cement leakage or ectopic cement injection (into the sacroiliac joint), migration, and embolization. Clinical data on the procedure is promising, demonstrating immediate improvement in mobility and significant pain relief in most patients undergoing the procedure [67, 72, 74, 76].
Embolization of sacral tumors is another useful therapy, which may be primary (single or repeat treatment sessions), adjuvant to surgical treatment, or palliative. Preoperative selective arterial embolization of hypervascular metastatic lesions reduces intraoperative blood loss and improves the surgeon’s ability to subsequently perform surgical resection. Studies have shown that embolization may cause tumor growth arrest, relieve pain, and reduce hospital stay [84–90]. The timing of preoperative embolization is also important. Typically, best results are achieved when surgery is performed within 24–48 h after embolization. Serial embolization can also be performed if there is persistent pain and/or evidence of progressive disease on imaging. It is typically performed in 4–6-week intervals until symptomatic improvement occurs or the tumor’s vascularity disappears. Complication rate is generally low. Risks of the procedure include nerve palsy, subcutaneous or muscle necrosis, postembolization syndrome (fever, pain, malaise), ischemic pain (usually transitory), infection, and tumor bleeding [87–90].
Over the last few decades, percutaneous ablation has emerged as an effective, minimally invasive, local treatment alternative to conventional methods, aiming to provide either palliation of painful bone lesions or local control of oligometastatic disease. Various image-guided ablation technologies have been applied to the treatment of bone metastases with varied levels of published evidence. Thermal ablation methods include radiofrequency ablation (RFA), cryoablation, microwave ablation, laser ablation, and more recently MRI-guided extracorporeal-focused ultrasound (MRgFUS) [91]. RFA and other ablation techniques are safe and reproducible, with promising results (pain relief 75–95%) [92]. Potential complications include skin burns and injury to heat-sensitive structures, such as nerves and bowel [93].
Finally, modern targeted medical therapy aims to control pain and reduce skeletal events of metastatic cancers. Such agents are mainly represented by bisphosphonates and denosumab. Bisphosphonates inhibit normal and pathological osteoclast-mediated bone resorption by direct inhibition of osteoclast activity by cellular mechanisms that affect osteoclast attachment, differentiation, and survival. They also reduce osteoclast activity indirectly, through effects on osteoblasts. In 2002, intravenous zoledronic acid was approved to treat patients with multiple myeloma and bone metastases from any solid tumor including prostate cancer. Denosumab is a human monoclonal antibody for the treatment of osteoporosis, induced bone loss, bone metastases, rheumatoid arthritis, multiple myeloma, and giant cell tumor of bone. It is designed to target RANKL (RANK ligand), a protein that acts as the primary signal to promote bone loss. Denosumab has been approved by the US Food and Drug Administration (FDA) for the prevention of skeletal related events in patients with bone metastases from solid tumors. Potential drawbacks of both drug families notably include the risk for osteonecrosis of the jaw and for the development of atypical femoral fractures [93–95].
Conflict of Interest Statement
No benefits have been or will be received from a commercial party related directly or indirectly to the subject matter of this article.
References
1.
Mavrogenis AF, Patapis P, Kostopanagiotou G, Papagelopoulos PJ. Tumors of the sacrum. Orthopedics. 2009;32(5):342.PubMed
2.
Unni KK, Inwards CY, Mayo Foundation for Medical Education and Research. Dahlin’s bone tumors: general aspects and data on 10,165 cases. 6th ed. Philadelphia: Wollters Kluwer Health/Lippincott Williams & Wilkins; 2010.
3.
Quraishi NA, Giannoulis KE, Edwards KL, Boszczyk BM. Management of metastatic sacral tumours. Eur Spine J. 2012;21(10):1984–93. doi:10.1007/s00586-012-2394-9.PubMedPubMedCentral
4.
Steinmetz MP, Mekhail A, Benzel EC. Management of metastatic tumors of the spine: strategies and operative indications. Neurosurg Focus. 2001;11(6):e2.PubMed
5.
Raque Jr GH, Vitaz TW, Shields CB. Treatment of neoplastic diseases of the sacrum. J Surg Oncol. 2001;76(4):301–7.PubMed
6.
Llauger J, Palmer J, Amores S, Bague S, Camins A. Primary tumors of the sacrum: diagnostic imaging. AJR Am J Roentgenol. 2000;174(2):417–24. doi:10.2214/ajr.174.2.1740417.PubMed
7.
Diel J, Ortiz O, Losada RA, Price DB, Hayt MW, Katz DS. The sacrum: pathologic spectrum, multimodality imaging, and subspecialty approach. Radiographics. 2001;21(1):83–104. doi:10.1148/radiographics.21.1.g01ja0883.PubMed
8.
Quraishi NA, Giannoulis KE, Manoharan SR, Edwards KL, Boszczyk BM. Surgical treatment of cauda equina compression as a result of metastatic tumours of the lumbo-sacral junction and sacrum. Eur Spine J. 2013;22(Suppl 1):S33–7. doi:10.1007/s00586-012-2615-2.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree