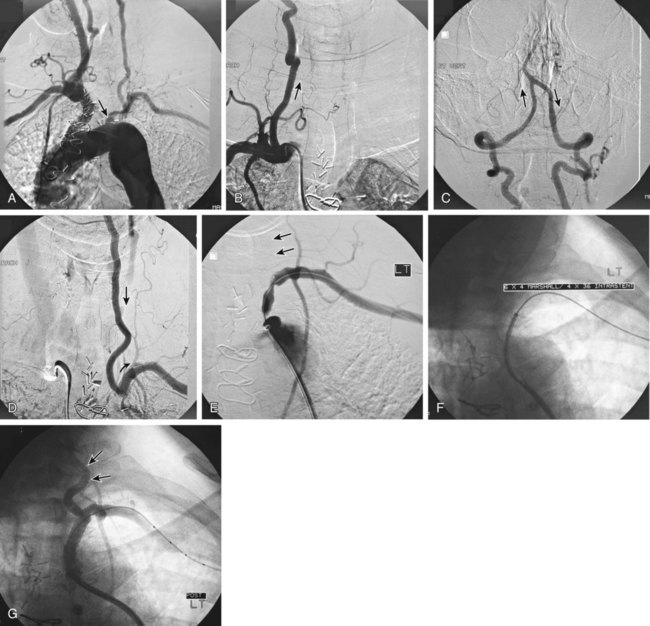
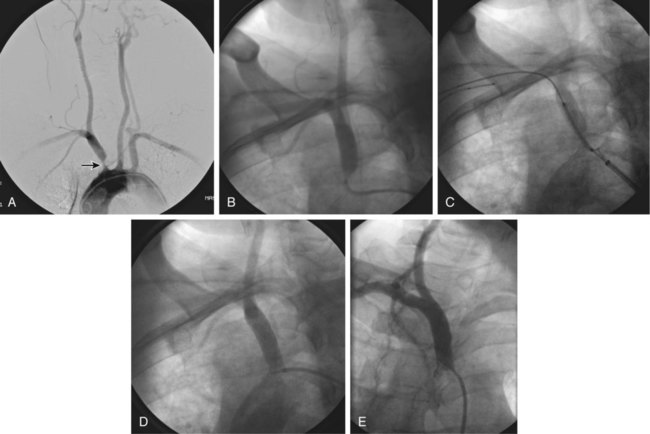
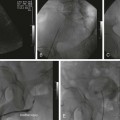
Symptomatic chronic ischemia of the upper extremity is commonly encountered in clinical vascular practice, comprising about 17% of symptomatic extracranial cerebrovascular disease1; 80% occurs in males. In contradistinction to chronic lower extremity ischemia, presenting symptoms in the upper extremity are often due to remote ischemia in the bed supplying collateral flow—namely, vertebral-subclavian and coronary-subclavian steal.2–8 This intimate association between the vascular territories of the arm, hindbrain, and heart (in post left internal mammary artery [LIMA] coronary artery bypass graft [CABG] patients) is unique and may present technical challenges and potential complications not typically encountered in other vascular beds. With modern techniques and equipment, however, operators treating underlying stenotic or occlusive lesions of the proximal upper extremity arteries by interventional techniques can expect high degrees of success with low complication rates.2,4,7,9–13 • 6F to 9F sheaths, 45 and 90 cm, long enough to extend through the area of intervention from the access site • Straight and angled hydrophilic wires • Diagnostic catheters of various shapes, such as Judkins right (JR)4, multipurpose, headhunter, LIMA, VTK, Amplatz, and Simmons • Straight or angled tip glide catheter • 0.035-inch exchange wire, atraumatic, straight, or J tipped • Balloons 4- to 10-mm diameter, 2 to 4 cm in length, with a long shaft length (110 cm long), if the site of access is from the femoral artery • Balloon-expandable stents capable of achieving these diameters, and lengths from 20 to 40 mm • Covered stents in similar sizes for treatment of restenosis or for emergency use in vessel perforations • Coronary stents in 3.0, 3.5, and 4.0 mm for vertebral or LIMA salvage (with compatible 0.014-inch wire) Symptomatic disease of the left subclavian artery is roughly eight to ten times more frequent than in the brachiocephalic trunk or right subclavian artery.9,10 This can be considered fortunate because intervention in the brachiocephalic or right subclavian artery naturally involves working in close proximity to the right common carotid artery, subjecting it to potential embolization, dissection, ostial compression, or stent coverage. Once guidewire access across the lesion has been achieved, one has the choice of whether or not to predilate before stent placement. Predilation has several advantages, including better visualization of the lesion length, especially of the distal stent landing point, proof of distensibility (especially in calcific lesions), and the ability to visually confirm correct balloon size. The main disadvantages are the possibility of propagating a dissection and, in theory, relieving the subclavian gradient enough to restore antegrade vertebral flow and lose the inherent protection against vertebral embolization retrograde flow provides. There is controversial evidence from a small study done over 20 years ago that restoration of antegrade flow may take several minutes,14 perhaps owing to chronic ischemic vasodilation in the arm. In our experience, however, restoration of antegrade flow in the vertebral artery occurs immediately on relief of the pressure gradient across the lesion, either with aggressive predilation or stenting. There is some evidence that direct stenting (i.e., without predilation) may be associated with a lower restenosis rate as well.10 In general, we gently predilate occlusions with undersized balloons to allow some antegrade flow for better visualization, and primary stent most stenoses that are not heavily calcified. As is the case with most aortic branch vessel disease, most subclavian disease is ostial or near ostial. This includes the right subclavian artery, where typically the lesion is at the brachiocephalic bifurcation. In general, these lesions have a high degree of resistance to dilation and subsequent recoil; accordingly, balloon-expandable stents are generally preferred to self-expanding stents or angioplasty alone.9,10 Many cases of stent migration before deployment in the lesion have been reported.7,12 This usually arises from either the stent edge catching in the lesion during attempted passage through the lesion, or the rear of the stent catching on the guiding catheter during attempted stent withdrawal if the stent cannot be passed across the lesion. In both instances, the stent may be loosened (“skimmed”) off the balloon and left free on the wire. Although there are several methods available to rescue this event, including safely deploying the stent in a more proximal vessel,7 this situation is best avoided. Stents that are factory rather than hand mounted may be more resistant to skimming, and the risk can be eliminated entirely by readvancing the dilator and sheath through the lesion before the stent is advanced. In this way, the stent can be positioned while it is still protected inside the sheath, which can subsequently be withdrawn, and the exposed stent deployed. Especially when using 6F guiding sheaths, the potential for distal embolization is minimized. Figures 34-1 to 34-6 illustrate various aspects of the interventions discussed.
Chronic Upper Extremity Ischemia and Revascularization
Subclavian and Brachiocephalic Disease
Equipment
Technique
Anatomy and Approaches
Technical Aspects

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Chronic Upper Extremity Ischemia and Revascularization