(1)
Department of Radiology Chair, Central State Medical Academy Department of Radiology, Moscow, Russia
4.1 Normal Breast
Currently MMG is accepted as a “gold standard” in the evaluation of breast diseases in patients older than 40. The ultrasound technique is widely used in young women for examination of the breast structure. ABVS opens a new approach to assessment of breast anatomy, with visualization of the whole gland at once allowing analysis of all observed changes in relation to other X-ray and MR modalities and good reproducibility of the corresponding images during follow-up.
Normal breast structure varies over time in the same women, during the menstrual cycle, pregnancy, after weaning, and during menopause [1–3]. This variability is also reflected in the ABVS images.
The female breast is composed of glandular, fatty tissue interspersed with fibrous or connective tissue. The distribution and prevalence of the fatty or glandular tissue depends on age and hormonal changes [4–8]. Thick fascia covering the pectoralis major and serratus anterior muscles make up the bed of the breast. Layers of premammary superficial and retromammary deep fatty tissue surround the gland. Chest and intercostal muscles are located posterior to the pectoral fascia. Fibrous septa extend from the dermis into the breast parenchyma to create the Cooper suspensory ligaments. They divide the gland into 15–25 cone-shaped functional lobes which are distributed in the fibrofatty stroma in multiple lobules. The lobes are arranged radially around the nipple with apices turned to it. The lobes drain into multiple ducts which coalesce and subsequently drain into lactiferous ducts which terminate at the nipple. The breast lobule consists of 20–40 acini or alveoli which are the main secretory unit of the breast tissue. The lobule and the first terminal duct arising from it constitute the terminal duct-lobular unit (TDLU). The TDLU is an important site for the development of cancer and benign proliferations of the breast [1].
The breast fibroglandular stroma is a clinically important structure consisting of supporting stroma and periglandular stroma. The supporting stroma is formed from processes of the superficial fascia and interlobular layers consisting of coarse connective tissue from collagen fibers, relatively poor in cells. The periglandular stroma immediately surrounds the ducts and has a loose soft fibrous structure, with abundant fibroblasts and several macrophages, lymphocytes, and plasma cells. The expression of the periglandular stroma is proportional to the development of the glandular tissue. The supporting stroma and periglandular stroma absorb X-rays equally and therefore they cannot be differentiated mammographically, appearing as continuous glandular tissue. The mammographic density of the breast depends on the amount of these stromal and epithelial elements. Increased MMG density is more apparent in young women than postmenopausal women.
In young women, the differences between supporting and periglandular stromata can be seen with the help of ultrasound. The supporting stroma appears on sonograms as areas of increased echogenicity. On the contrary, periglandular stroma is seen as low echogenicity areas. These two structures should be considered as glandular tissue during ultrasound imaging analysis to enable comparisons between MMG and US. Thus, breasts in the early reproductive period (at the age of 15–25) have a cellular structure with alternating zones of low and high echogenicity. This variability of the echostructure is provided by the periglandular stroma. The structure of the glandular tissue varies from reticular to cellular in young women with a regular menstrual cycle. Edema and hyperemia of the periglandular stroma decrease echogenicity and provide a reticular pattern in the secretory phase. The periglandular stroma narrows, and the echogenicity of the glandular tissue increases with less expressed nodularity in the proliferation phase. The echostructure becomes cellular. The additional hypoechoic masses are identified more easily against this background in the proliferation phase (Figs. 4.1 and 4.2). Therefore, the proliferative phase of the menstrual cycle also is preferable for examining the breast with 3DUS in the reproductive period, as in conventional 2D ultrasound. Collapsed ducts are not clearly visible in the proliferative phase against a background of echogenic glandular tissue. They can sometimes be seen in the secretory phase of MC as hyperechoic dots or lines.



Fig. 4.1
A case of normal breast in a 20-year-old woman, without a history of pregnancy. Proliferation phase. Hyperechoic glandular tissue. Cellular pattern of breast structure due to the balanced development of supporting and periglandular stroma. (1) Premammary fat, (2) glandular tissue, (3) nipple and areolar area, (4) retromammary fat, (5) superficial fascia, (6) pectoral muscles. Comparison of data of the same patient in conventional 2D HHUS and ABVS images. (a) HHUS image with a small field of view depicting part of the gland, (b) ABVS tomogram in R LAT (latero-medial) view of the right breast. The rectangle marks the nipple area. ABVS showed close to “anatomical” visualization of the breast structures

Fig. 4.2
Breast in the proliferation phase of the menstrual cycle in a 30-year-old woman without a history of a pregnancy. Note the good contrast between the glandular tissue and the lesion (fibroadenoma). Comparison of data for the same patient in conventional 2D HHUS and ABVS images. (a) HHUS image depicting the glandular tissue in a line-structure pattern. (b) ABVS tomogram in R LAT (latero-medial) view of the right breast. Hypoechogenic fibroadenoma (arrow) in the upper part of the breast is clearly visible against the background of highly echogenic glandular tissue which is more prominent than the fatty tissue. Rectangle marks the nipple
Glandular tissue occupies practically all areas of the gland in the early reproductive period and can be clearly seen in the anterior and posterior slices of a full-sized image (Fig. 4.3). Adipose tissue is expressed minimally. Cooper’s ligaments cannot be clearly visualized on the background of this high echogenic glandular tissue. The nipple is represented as a hyperechoic ovoid structure with a minimal hypoechoic distal shadow.


Fig. 4.3
Breast in the secretory phase of the menstrual cycle in a 28-year-old woman, nulliparous. Thickened hypoechoic periglandular stroma (2). The anechoic structure inside the stroma corresponds to the slightly dilated ducts (1) in the secretory phase of the menstrual cycle. Comparison of data of the same patient in conventional 2D HHUS and ABVS images. (a) HHUS image depicts thickened glandular tissue and a decrease in echogenicity of the breast with ill-defined line-structures. (b) ABVS tomogram of the left breast. L AP—left anteroposterior coronal slice. Uniform decrease of echogenicity of the breast parenchyma. Small anechoic areas correspond to enlarged ducts
The structure of the right and left breasts is basically quite symmetrical. If any asymmetry is present, pathology should be excluded [3]. This was stated in relation to MMG but can be applied to ABVS as well. Computer workstations allow assessing the right and left breast by matching slices. These images and saved clips from the whole array of ultrasound data facilitate identification of developmental abnormalities, the preferential location of the glandular tissue, its distribution per quadrants, its structural features, and pathological masses (Fig. 4.4). The adaptive mode offered by ABVS—nipple shadow reduction tool—improves visualization of the retroareolar structures. 3D data should be acquired using the nipple maximum lateralization technique; it helps to reduce the “no-show” areas under the nipple and retroareolar region on LAT (latero-medial oblique) and SUP (superior craniocaudal) views. With a conventional 2D US technique, the visualization of retroareolar regions without informational and quality loss usually requires the additional use of a water paddle.




Fig. 4.4
Analysis of the breast structure using ABVS data on a workstation and US device. (a) ABVS workstation side-by-side comparison of the right (left part of picture) and the left (right part of picture) breast in coronal anteroposterior views at the level of the glandular triangle (20 mm in depth from the nipple). The type of view is indicated by the icon. The nipple is marked in the center of each breast by a rectangle. The structure of both breasts is symmetrical lobulated with a medium amount of fatty tissue. Glandular tissue is distributed around the nipple equally. The region under the nipple is seen without “no-show” zones. (b) ABVS multislice mode analysis on a US device. R MED view (mediolateral oblique view) of the right breast. The depth of each slice is marked from 75 to 84 mm from the medial part to the lateral part. Fifty-six slices were obtained with a slice thickness of 3 mm. These four images (25/56, 26/56, 27/56, 28/56 slices) were selected to show the structure of the middle part of the breast, and they could be compared with corresponding slices in digital breast tomosynthesis. The nipple is marked by a rectangle. Note a hypoechoic area in the retroareolar region. Slight cellularity of the glandular tissue corresponds to the early reproductive type of the breast
The most intensive breast development starts at the end of pregnancy and in lactation. During pregnancy, the breasts are influenced by hormones produced by the placenta, with hyperplasia of glandular lobes and increase development of ducts. At the end of pregnancy, the breast loses the cellular structure and appears as a uniform thickened layer of glandular tissue. In this period, TDLU transform and develop further, connective tissue septa are stretched and thinned, and edema and increased vascularity are present. Echogenic layers of supporting stroma are correspondingly thin on a sonogram. ABVS shows these changes as a loss of reticular structure, even a decrease in echogenicity of the glandular tissue, with several subtle hyperechoic linear structures (ducts), mainly in areolar areas (Fig. 4.5). After lactation, the breast lobules involute following the withdrawal of prolactin stimulation. On ABVS, cellular or reticular breast structure is restored.


Fig. 4.5
Breast during lactation. Comparison of data for the same patient in conventional 2D HHUS and ABVS images. (a) On an HHUS image, the absence of pre- and retromammary fat tissue is noted. An absence of cellularity, a uniform decrease in echogenicity of the glandular tissue. The absence of interlobular fat and fibrous tissue. Linear horizontal stripes indicate the milk ducts. (b) ABVS image from L-axilla view (axillary oblique view of the left breast). The breast consists only of glandular tissue of low echogenicity. Note the homogeneity of the breast parenchyma during lactation
At the age of 35 or sometimes earlier, fat tissue appears in between the glandular tissues, even in lean-bodied women. Areas of the breast between the fat lobes mostly still have a cellular structure. Later, at the age of 40, the proportion of fatty tissue is further increased, and the glandular tissue loses its cellular pattern due to interlobular fibrosis (Figs. 4.6, 4.7, and 4.8).




Fig. 4.6
Breast structure in the late reproductive period in a 36-year-old woman 1 year after pregnancy and lactation. Comparison of data for the same patient in conventional 2D HHUS and ABVS images. (1) Premammary fat, (2) glandular tissue, (3) nipple and areolar area, (4) retromammary fat, (5) superficial fascia, (6) pectoral muscles. (a) HHUS image shows disappearance of cellularity of the breast tissue and increase in echogenicity due to involution process in the stroma. Enlargement of pre- and retromammary spaces. (b) ABVS tomogram of the left breast obtained in the LAT view (left latero-medial oblique view). Increased echogenicity of the breast tissue and prominent fatty tissue in the premammary region

Fig. 4.7
Breast structure in the late reproductive period in a 45-year-old female without a history of lactation. Comparison of conventional 2D HHUS and ABVS images of the same patient. Key: (1) premammary fat, (2) glandular tissue, (3) nipple and areolar area, (4) retromammary fat, (5) superficial fascia, (6) pectoral muscles, (7) Cooper’s ligaments. (a) On HHUS image stroma is echogenic with areas of preserved cellularity. Premammary fat space is more prominent than in the early reproductive period. (b) ABVS tomogram of the left breast obtained in the AP view (coronal anteroposterior view). The distribution of glandular tissue is located mainly in the upper quadrants, with fatty tissue dominating in the lower quadrants

Fig. 4.8
Involution changes of the breast in a premenopausal 48-year-old woman. Comparison of the HHUS and ABVS data of the same patient. (1) Premammary fat, (2) glandular tissue, (3) nipple and areolar area, (4) retromammary fat, (6) pectoral muscles, (7) Cooper’s ligaments. (a) On the HHUS image reduction and narrowing of the echogenic glandular tissue, increase of fibrosis and appearance of fatty lobes interspersed in the residual glandular tissue are clearly seen. (b) ABVS tomogram of the left breast obtained in AP view (coronal anteroposterior view). 1 The whole-breast structure is shown with a distribution pattern of echogenic glandular lobes interrupted by fatty lobules. Thin strip lines of residual stroma are seen as hyperechoic lines
At the age of 50–60, the proportion of fatty tissue in the breast increases even more. The breast tissue on MMG shows as transparency. This transparent background on MMG allows clear visualization of microcalcification and any additional lesions. With ABVS, it is also possible to show this fatty degeneration: the gland appears evenly as a lobular structure of reduced echogenicity with several cord-like elements of high echogenicity, representing the thinned glandular tissue and fibrosis of supporting stroma. Usually the residual glandular and fibrous tissue is preserved in the upper-outer and central areas, while the structure of internal and lower quadrants is composed mostly of fatty tissue which is of reduced echogenicity (Fig. 4.9).


Fig. 4.9
Fat involution of the breast in a postmenopausal 56-year-old woman. Comparison of the HHUS and ABVS data for the same patient. (1) Premammary fat, (2) glandular-fibrous tissue, (3) nipple and areolar area. (a) HHUS image depicts the prevalence of fat tissue and thin strips of glandular tissue with fibrotic changes. (b) ABVS tomogram of the right breast obtained in R LAT (latero-medial view). 2 Residual glandular and fibrous tissue in the upper and central regions. In the lower ones fat tissue prevails
The most typical ABVS features of the breast structure at the age of 60–70 and above are an islet or narrow strips of fibrous tissue of high echogenicity and sparse ducts in the nipple and areolar areas with fatty lobules of lowered echogenicity constituting the prevalent part of the gland tissue (Fig. 4.10). So with ultrasound, we have similarity of representation compared to MMG of the fatty and fibrous tissues. As ABVS covers the whole gland in the same projections as in MMG, it makes the ABVS images look like mammograms. Adipose tissue is hypoechoic on the sonotomogram, similar to on a mammogram. Glandular tissue and fibrosis are hyperechoic or have a denser background, as on a mammogram.
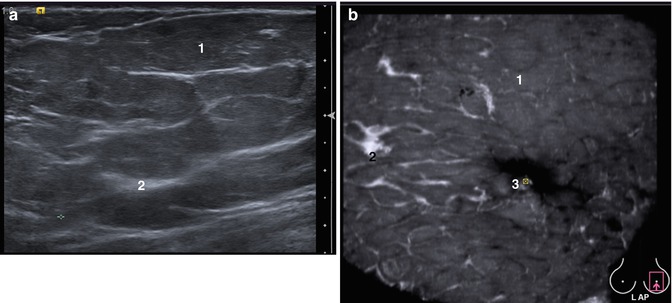

Fig. 4.10
A case study of the breast with total fatty involution in a 67-year-old woman. Atrophy of the glandular tissue. The predominant tissue is fat with a small amount of fibrous septa. Comparisons of HHUS and ABVS data for the same patient. (1) Premammary fat, (2) fibrous tissue, (3) nipple and areolar area. (a) HHUS of the fatty involute breast. (b) ABVS tomogram of the left breast obtained in AP view (coronal anteroposterior view). Uniform fatty structure of reduced echogenicity of the whole gland is seen on the coronal view
In some cases, the cellular structure of the stromal-glandular complex is preserved significantly longer, especially in lean-bodied women with a history of a long lactation period (up to 1 year) (Fig. 4.11). About 40 % of postmenopausal women have a significantly dense breast. Hyperestrogenism forms a background for the decreasing involution changes in the glands during this period. Uterine leiomyoma and endometriosis are accompanied by hyperestrogenism and hormonal imbalance, leading eventually to the stimulation of ductal and glandular epithelial cell proliferation, thus prolonging the beginning of breast involution (Fig. 4.12). Hormone replacement therapy also slows down the processes of fatty involution (Fig. 4.13).




Fig. 4.11
Breast structure in a 76-year-old woman with a history of functional giant ovarian fibroma with estrogen production. The tumor caused benign transformation of the breast epithelium and slowness of the involution process. Comparison of the images obtained from HHUS and ABVS. (1) Premammary fat, (2) glandular tissue, (3) nipple and areolar area. (a) HHUS registered an increased amount of glandular tissue, not corresponding to the patient’s age. (b) ABVS tomogram of the left breast. L LAT view (left latero-medial oblique view). Hypertrophied glandular and fibrous tissue in the upper and lower areas are clearly visualized

Fig. 4.12
Breast structure in a 52-year-old female who has undergone hormonal replacement therapy for 7 years. Benign fibrocystic changes in the breast. HRT slows down breast involution. Comparison of HHUS and ABVS data in the same patient. (1) Premammary fat, (2) fibrous tissue, (3) nipple and areolar area, (4) cyst. (a) HHUS shows thick glandular tissue with areas of benign fibrocystic changes. (b) ABVS tomogram of the right breast. R MED view (mediolateral oblique view). Glandular tissue with slight cellularity, no fatty involution present

Fig. 4.13
Breast structure in a 58-year-old woman with leiomioma and diffuse endometriosis. Benign fibrocystic changes with significant glandular hyperplasia. Comparison of HHUS and ABVS. (2) Glandular-fibrous tissue, (3) nipple and areolar area, (4) cyst. (a) HHUS clearly depicts the increased echogenicity of the thickened glandular tissue and enlarged ducts. (b) ABVS tomogram of the right breast. R LAT view (latero-medial oblique view). Note an absence of pre- and retromammary fat; the reticular structure of the gland is caused by the dilated ducts. The cyst lies close to the nipple area
X-ray mammography is almost incapable of detecting cancer against the background of dense glandular tissue. Meanwhile, high-density breast does not preclude the identification of masses by ABVS but rather enhances it [9, 10]. Therefore, ultrasound, and even better ABVS, should be present in the algorithm for screening women with dense glandular tissue (types ACR III and IV according to BI-RADS classification).
4.2 Fibroadenomas
The fibroadenoma (FA) is the most common benign breast tumor, occurring frequently in the second and third decades and being reported as a common breast lesion also in postmenopausal women who fall into the screening age group [11, 12]. Overall fibroadenomas comprise 50% of all breast biopsies, and the rate increases to 75 % for biopsies in women under the age of 20 [13]. FA often occurs as a reaction to hormonal changes. Hyperestrogenemia contributes to its development. Usually it presents as a palpable non-tender mobile lesion 1–2 cm in diameter or can be detected incidentally by MMG especially in postmenopausal women. In some patients (10–20 %), they can be multiple, bilateral, or unilateral. Some FAs (especially giant FAs) can demonstrate rapid growth in adolescent girls, or during pregnancy and lactation causing asymmetry and discomfort, and thus require surgery [14]. Complex FAs are associated with an increased risk of subsequent breast cancer. Observational and molecular studies suggest FAs can transform into phyllodes tumors, which are graded as benign, borderline, and malignant.
On FNAC or needle biopsy, FAs arise from terminal duct-lobular units, the same functional unit for lobular and ductal carcinoma. An FA contains benign epithelial cells (myoepithelial and luminal). As it grows older, it becomes hyalinized, associated with calcification or undergoes myxoid degeneration. Histologically, several types of FA are defined: intracanalicular, pericanalicular, and mixed. Pericanalicular fibroadenomas are predominant in women under 45 and represent an overgrowth of connective tissue around the breast duct. Intracanalicular ones are characteristic for women over 50 and are formed inside the duct gland.
4.2.1 A Typical Fibroadenoma
Fibroadenomas are diagnosed by different methods—MMG, US, and MRI. Ultrasound is useful in diagnosis of FA. Typical US features of FAs are present in only 20–30 % of cases [15]. FAs are more prevalent in young women with dense breasts in whom MMG can be negative. The US appearance of FAs includes well-circumscribed, round, or oval hypoechoic masses, slightly lobulated, with clearly defined margins, and a vessel on the periphery of the lesion or round pattern of vascularity. In hyalinized FAs, the lesion can demonstrate weak acoustic shadowing; in calcified FAs, the shadowing becomes more prominent.
Screen-detected FAs on MMG exhibit a well-circumscribed, round, or oval mass with or without lobulation, characteristically demarcated from the surrounding tissue to create a halo [16]. This is present in 98 % of FAs and is caused by a radiolucent rim around the periphery of a lesion resulting from atrophy of the surrounding tissue with its replacement with fat [17]. Pericanalicular FAs demonstrate this rim on MMG more prominently than intracanalicular FAs.
The ABVS image of an FA is typical without any significant dependence on histological structure. Intracanalicular FAs are often hypoechoic and homogeneous by structure, with a clear prominent hyperechoic rim or “compression” sign. Pericanalicular FAs have a more significant echogenicity, lobulated structure, and also clear capsule or rim (Fig. 4.14). This compression sign, which is highly specific for FAs, on ABVS may be a distinctive pattern from malignant lesions which have a retraction pattern. This sign is equally well noticed in all FAs regardless of histological type in all views (Fig. 4.15).



Fig. 4.14
A case of intracanalicular fibroadenoma in the left breast in a 47-year-old woman. Comparison of the data of HHUS, ABVS, and MMG. (a) HHUS. Fibroadenoma is presented as a clearly defined hypoechoic mass well demarcated from the echogenic glandular tissue. (b) ABVS. L LAT view (left latero-medial oblique view). The whole breast is seen with ABVS. A hypoechoic well-circumscribed nodule with an echogenic rim (which indicates benignity) in the upper part of the left breast can be detected (white arrow). The nipple is marked by a rectangle. (c) These mammograms (CC and MLo) of the left and right breasts are presented simultaneously for comparison. An ovoid dense mass with clear contours is visible in the upper-outer quadrant of the left breast in mediolateral oblique and craniocaudal frontal views. The lesion is marked with a white arrow

Fig. 4.15
A case of a large fibroadenoma (sized 20*9 mm) in a 35-year-old female with a dense breast. Comparison of HHUS and ABVS data. (a) HHUS shows a hypoechoic oval-shaped mass with clear smooth contours. (b–d) On ABVS obtained in different views. (b) L MED view (left mediolateral oblique view), (c) L AP view (left anteroposterior coronal view), (d) L SUP view (left superior-to-inferior view). “Dense breast” on ABVS is seen as echogenic cellular breast tissue corresponding to the age. Fibroadenoma in the lower-inner quadrant of the left breast, according to L SUP and L MED slices, is clearly visible on this “dense” background. A hyperechoic rim, which surrounds the FA, is thin but clear. The nipples are marked by rectangles
4.2.2 Multiple Fibroadenomas
Multiple FAs have been reported in families raising the possibility of familial predisposition. In a nonfamilial setting, multiple FAs, especially with myxoid degeneration, can enlarge in patients on follow-up leading to excision, on suspicion of malignancy [18]. The possibility of MMG to detect multiple FAs depends on their size, the presence of calcification, and the breast type (according to ACR).
Mammography shows the distinct boundary of a fibroadenoma located inside the fat tissue. It may be rather difficult to identify the same FA on a background of fibrocystic disease or dense breast. In this case, it is necessary to use the capabilities of ultrasound. US is superior to MMG due to better contrast between the FA and stromal-glandular complex. In our study, MMG failed to show a large FA in the upper quadrants inside dense breast tissue but clearly revealed only a small one—in the lower quadrants where fatty tissue dominates. Being a large one, the FA, which was approximately 2 cm in size, was occult for MMG but clearly visible for US. On side-by-side comparison of corresponding MMG and ABVS images, the large FA is masked by dense glandular tissue (Fig. 4.16). It is really difficult some times to follow up patients with multiple benign lesions. But the importance of excluding malignancy is of priority in a high-risk group of patients. In this way, the use of ABVS can help to depict all the lesions throughout the breast with better topography, size measurements, and follow-up for comparison. And in doubtful cases, the ABVS hyperechoic rim sign around multiple fibroadenomas adds diagnostic information to exclude malignancy (Fig. 4.17).






Fig. 4.16
A case of multiple fibroadenomas with an occult fibroadenoma in the upper quadrants in a 51-year-old patient. Comparison of the HHUS, ABVS, and MMG appearance. (a) HHUS. Hypoechoic mass with nodularity and clear margins is seen in the glandular tissue. (b) ABVS. RAXIL view (axillary view). ABVS shows two fibroadenomas in one breast in the whole gland view: one is large, and the second is small. One can be seen on the border of the upper quadrants (thick arrow) and the second located in the lower-inner quadrant (thin arrow). The nipple is marked by a rectangle. The residual glandular tissue is located in the upper quadrants at the 10–2 o’clock position. In other areas fatty tissue predominates. (c) A side-by-side comparison of the corresponding images of MMG (left part) and ABVS data (right part). MMG clearly shows only one fibroadenoma in the lower area of the breast (thin arrow). Dense glandular tissue with streaking and fibrosis disguises a second larger fibroadenoma. ABVS displays the fibroadenoma very clearly due to contrast with glandular tissue (thick arrow). Both fibroadenomas are surrounded by a hyperechoic capsule. (d) Image interpretation on an ABVS workstation. Multiplanar reconstruction of axillary view across a large fibroadenoma. In the left part, a coronal view of the right breast with two lesions appears; at the top of the picture, a typical longitudinal view of the lesion is presented and at the bottom a pictogram with the indication of the lesion’s location at the 12 o’clock position, with an indication of the distance from the skin to the mass (1 cm) and from the lesion to the nipple (5 cm)


Fig. 4.17
A case of multiple old fibroadenomas with a large one in the axillary recess in a 48-year-old woman simulating a multifocal cancer with metastasis in axillary lymph node on a screening mammogram. Comparison of HHUS, ABVS, and MMG performance. HHUS showed multiple hypoechoic lesions with irregular margins and a slight distal shadowing. The biggest mass (open arrow) was found in the axillary recess simulating an enlarged lymph node with cancerous changes. Increased superior-to-inferior diameter is noticed in the small lesion. (1) All the lesions demonstrate increased stiffness on sonoelastography, especially the large one (open arrow). (2) Side-by-side comparison of mammogram in MLo view (left part) and ABVS image (right part) in the corresponding R MED slice. The lesion on the MMG is dense (white thin arrow) with a big one thought to be a lymph node (open arrow) in the axillary region. In the same region, a hypoechoic lesion with a typical for fibroadenoma hyperechoic rim is noticed (thin arrow) on ABVS. (3) Side-by-side comparison of mammogram in CC view (left part) and ABVS image (right part) in the corresponding R SUP slice. The lesions on the ABVS tomogram* show a clear rim around them; no retraction sign is seen (*ABVS tomogram is not rotated clockwise)
4.2.3 Calcified Fibroadenoma
Usually old FAs contain calcifications resulting from degenerative processes. An indicative sign of a long-standing FA is calcified deposits. Long-term observation may show a gradual increase in the number and size of calcifications. They are formed in areas of necrosis or hyalinized stroma and can be of very intricate form. A calcified FA, even a tiny one, and on a background of dense tissue, can now be easily detected by X-ray. The calcification of FA can be complete, coarse, popcorn-like bizarre calcification, evolving calcification, linear, punctate, granular, or pleomorphic [19]. The appearance of small-sized grouped calcifications on an MMG is highly suspicious in terms of malignancy and requires a biopsy, whereas a uniform benign calcification tends to require follow-up. US is inferior to MMG in depicting microcalcifications but clearly shows macrocalcifications. A distal shadowing appears behind the calcified structures. Using ABVS, it was reported that the compression sign or hyperechoic rim allows detection of the benignity of the lesions with high sensitivity and specificity [20]. The benign node on ABVS is surrounded by a thin hyperechoic capsule (Fig. 4.18).


Fig. 4.18
A case of an old intracanalicular fibroadenoma of the left breast with hyalinosis of the stroma in a 55-year-old woman. Comparison of the ABVS and MMG performance. (a) Image analysis on the ABVS workstation. L LAT view (left latero-medial oblique view). The nipple is marked by an open arrow. A hypoechoic nodule with clear contours and a rim on the background of the residual glandular tissue in the central area (thin arrow). (b) Left breast mammogram in a craniocaudal direct view. Dense nodule with clear contours in the same nipple-areolar zone (arrow)
4.2.4 Phyllodes Tumor
Phyllodes tumor is a rare lesion which accounts 0.3–0.9 % of all breast tumors, with peaks between 45 and 49 years. It is characterized by rapid growth and large size; in some cases, it can occupy from one-half to three-fourths of the breast, varying from 2–3 to 10–20 cm in diameter [21]. The mass is non-tender, mobile, and well circumscribed. Some patients with phyllodes tumor had a past history of fibroadenoma. This tumor is characterized by a proliferation of stromal elements separated by epithelium to give rise to the frond or leaflike structures which differentiate a phyllodes tumor from a fibroadenoma. The degree of stromal hypercellularity, cytological atypia, and stromal overgrowth varies from absent in benign and low in borderline to high in malignant. The accuracy of cytological diagnosis ranges from 40 to 80 % and is not dependent on the histological structure of the tumor [22]. A low-malignancy tumor (high degree of histological differentiation) is microscopically similar to an FA and differs only by cellularity and increased mitotic activity.
Radiographically a phyllodes tumor is more typically characterized by sharp polycyclic contours and lobular structure. The signs are nonspecific. Calcifications are rare. The US picture is characterized by the appearance of fluid-filled elongated spaces or clefts inside the solid hypoechoic mass with well-defined contours. Large nodes have a lobular structure and marked hypervascularity. The ABVS picture is nonspecific and very similar to fibroadenoma (Fig. 4.19).


Fig. 4.19
Sonographic appearance of a benign phyllodes tumor in the right breast in a 28-year-old woman proved only after lumpectomy by histopathology. Comparison of HHUS and ABVS data. (a) HHUS demonstrated a large well-circumscribed hypoechoic lobulated mass with clear contours and small focus of anechoic cystic focuses inside (arrows) on the background of well-defined glandular tissue. FNAC found fibroepithelial cells, and histopathology has reported clefting growth pattern with variable cellularity with no mitotic activity or cytological atypia. (b) ABVS image obtained in R AP (anteroposterior coronal view). ABVS shows a hypoechoic mass (arrows) that causes the displacement of the glandular tissue (1) in the upper-external quadrant of the right breast. The signs are nonspecific
Suspected phyllodes tumors are better diagnosed by MRI. They are usually of homogenous high signal intensity on T2-weighted images and demonstrate rapid enhancement on dynamic contrast-enhanced MR images.
4.2.5 Atypical Fibroadenoma
A mammographic picture of a fibroadenoma often resembles those of a cyst or medullary cancer. The differential diagnosis in these cases is rather demanding. Speculation about cancer or malignant transformation of fibroadenoma is reasonable if you detect roughness of contour of the node in any place, as well as a breakthrough of the rim of clarification. The tumor may have lobulated structure, indistinct boundaries, and heterogeneous structure. Differentiation of this mass from a malignant one is sometimes difficult using both US and mammography. Mammography can change the visualization of the nodule due to the varying compression force during imaging or due to growth of the mass. Ultrasound and sonoelastography are not always convincing. Old fibroadenomas can be dense. But unlike a malignant lesion, they do not form spicules and retraction phenomenon on an ABVS tomogram, which simplifies the differential diagnosis in complex cases (Fig. 4.20).
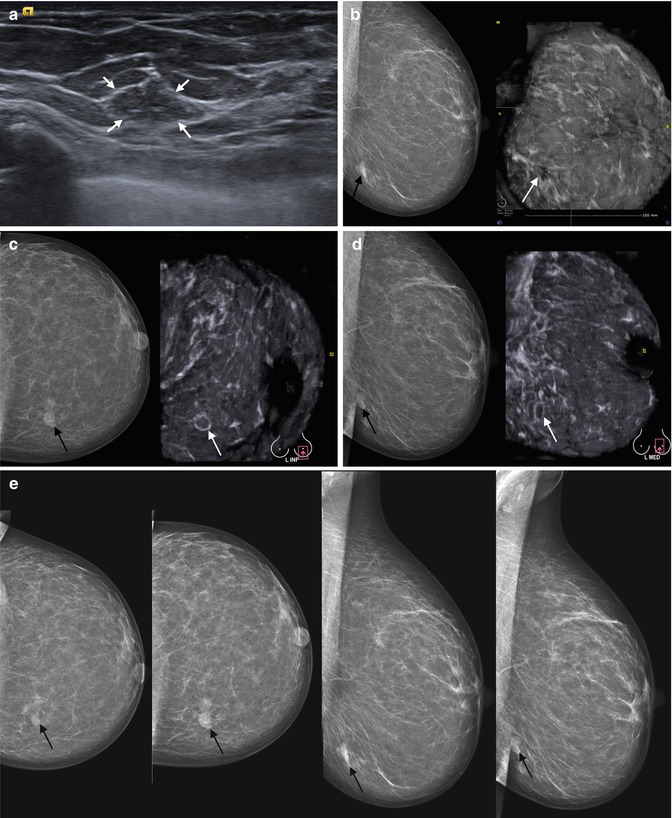

Fig. 4.20
Atypical fibroadenoma with growth changes in the lower-internal quadrant of the left breast in a 44-year-old female. Follow-up study. Comparison of HHUS, ABVS, and MMG performance. Excision with histopathology proved the diagnosis of fibroadenoma without atypia. (a) HHUS shows an isoechoic lesion poorly differentiated from the background of adipose tissue. (b) Side-by-side comparison of the corresponding images of MMG (left part) in MLo view and ABVS image (right part) in L MED view (left mediolateral oblique). The mass is considered with both techniques as an area of fibrosis. No retraction phenomenon is noticed in the area of the lesion. (c, d) Control study 3 months later. Side-by-side comparison of the corresponding images of MMG (left part) in CC view (c), MLo view (d) and ABVS image (right part) in L SUP (c) view, L MED view (d). The lesion has changed on MMG (black arrow). On ABVS it resembles a fibroadenoma with hyperechoic capsule (white arrow). (e) Series of comparative X-ray mammograms of the left breast with a 3-month interval. The changes in the dense pattern and contours are visible during follow-up
4.2.6 Dynamic Monitoring for Fibroadenomas with 3D ABVS
Follow-up is indicated in fibroadenomas under conservative treatment. Some lesions can multiply and some progress in size with hormonal changes and several other conditions. Monitoring with US is preferable because of the absence of X-ray exposure. One can trace the growth of fibroadenomas examining their shape, documenting the exact localization of the masses, and measuring them. Reproducibility of a new method is an important factor in follow-up studies. However, HHUS is an operator-dependent technique with no possibility for independent second reading. Follow-up with ABVS resolves the tasks of independence and reproducibility. An ABVS tomogram meets these requirements. The ABVS technique is reproducible, as confirmed by publications and our research [23, 24]. ABVS data are more reliable because the scan is carried out in automatic mode; dependence on the operator and the subjectivity of the assessment are excluded.
In the presented case, the patient had a fibroadenoma in the axillary process of the breast with no growth during the follow-up, but an additional small-sized fibroadenoma appeared 12 months later in the same breast, which was documented using ABVS. The absence of a small fibroadenoma in the previous study was confirmed objectively by retrospective analysis of the 3D data array from comparable views (Fig. 4.21).


Fig. 4.21
Follow-up study of a fibroadenoma with ABVS in 27-year-old female with a history of a pregnancy and lactation 2 years ago. Comparative ABVS study of the same patient 1 year later. ABVS L LAT scans of the left breast. (a) Initially a single fibroadenoma is presented as a hypoechoic mass in the axillary process of the breast (arrow) on the background of well-defined glandular tissue. (b) One year later a second fibroadenoma appears in the left breast (short arrow). The first one is seen in the same quadrant and in the same o’clock topography
Fibroadenomas more often require determination of topography of the mass to conduct a needle biopsy, so the use of ABVS in these cases is also preferred.
4.3 Breast Cystic Disease
Benign fibrocystic change is a rare condition before the age of 20 and becomes more prevalent in perimenopausal women, and microscopic lesions persist in postmenopausal women [25]. Cystic formation is most common in patients with benign epithelial proliferations and also may present in “normal” breast tissue. Benign epithelial proliferations associated with fibrocystic change include intraductal papillomas, fibroadenomas, duct ectasia, radial scars, sclerosing adenosis, epithelial hyperplasia of the usual type, and many more. Large cysts, fibroadenomas, and duct ectasia are classified as local forms of benign fibrocystic change. Diffuse forms can be represented by predominant hyperplasia of glandular tissue (sclerosing adenosis or fibrosclerosis). Diffuse and local changes can combine.
Several hormonal abnormalities have been implicated in the pathogenesis of fibrocystic change, including hyperprolactinemia, increased estrogen levels, reduced progesterone levels, and excess thyroid hormone activity [26]. The most pronounced forms of epithelial hyperplasia and cystic formation occur in patients with endometriosis. Hyperandrogenism is accompanied with a predominance of stromal fibrosis.
Apocrine epithelium is an inherent feature of fibrocystic change, and the presence of apocrine cells in a breast biopsy is generally considered by pathologists to be a reassuring feature of benignity. Apocrine metaplastic change develops in the terminal duct-lobular unit. Acini dilate because of fluid production by this apocrine metaplastic epithelium. Several adjacent acini have fused to form a larger cystic space or multi-chambered ones [27]. There is molecular evidence that some benign forms of apocrine epithelium may be potentially neoplastic [28].
Fibrocystic change and simple cysts are generally not difficult to diagnose. Usually they present in association with other screen-detected lesions.
4.3.1 Atypical Breast Cysts
Cysts can appear as round, oval, or well-circumscribed masses. Their contours are smooth and sharp. A radiolucent rim can be seen around the cyst, particularly a large one, narrow and smooth in contrast to that in cancer. Dense breasts can mimic cysts mammographically. And in fatty breasts, the cysts can exhibit the halo mark [29]. Small cysts are usually indistinguishable mammographically. The exception is the presence of calcium deposits within the cysts. Calcification is not a common feature.
Ultrasonography is the most accurate diagnostic modality in assessing cysts. Both small and large cysts are clearly visible using US. All of them have a typical appearance. Most often they are ovoid or round shaped with an anechoic content, with clearly differentiated internal and external contours, and distal enhancement [30, 31]. Sometimes cysts may require aspiration or biopsy to clarify their nature. In these cases, topographic mapping is needed. After aspiration of a cyst, follow-up US with or without MMG should be performed within a period of 4–6 weeks.
Cysts on ABVS coronal slices are found as anechoic round or oval clearly separated “cavities” without a hyperechoic rim if uncomplicated (Fig. 4.22). Fibrocystic changes are marked by the wall thickening, lumen enhancement, and roughness of contours of the ducts. “Pocket”-shaped ectasias are often detected as hypoechoic areas along the course of the main duct. These duct dilations are difficult to differentiate from cysts. ABVS can also display small cystic areas. They have a honeycomb appearance (Fig. 4.23). Dyshormonal hyperplasia can cause an increase in echogenicity of the parenchyma due to alternating hyperechoic connective tissue elements with hypoechoic glandular structures.



Fig. 4.22
Comparison of HHUS, MMG, and 3D ABVS data in the case of fibrocystic change of the breast in a 52-year-old female patient. Multiple cysts visualized ((1) cysts, (2) breast tissue, (3) premammary fat, (4) pectoral muscles). (a) HHUS. Multiple large cysts and cystic conglomerates are seen within the breast tissue. Siescape panoramic view of the breast. (b) MMG of the left breast. Multiple rounded opacifications in the dense breast. (c, d) ABVS tomogram of the left breast. (c) L AP slice—left anteroposterior coronal view. (d) L LAT view—left latero-medial oblique view. Black “holes” present in all regions on the backdrop of well-preserved glandular tissue

Fig. 4.23
Comparison of HHUS and ABVS performance is shown in the case of fibrocystic changes with local areas of duct ectasia in a 42-year-old woman. (a) HHUS demonstrates the local area of multiple cystic lesions. (b) ABVS tomogram of the right breast obtained in the AP view (anteroposterior coronal view) depicting the zone of duct ectasia (circle) in the division of inner quadrants
4.3.2 Atypical Cysts
Complex cysts are thick walled; may have thick septa, intracystic masses, or other solid components; and can also merge into a conglomerate. Clustered multiple cysts typically result from destruction of the separating septum to form a multi-chamber cystic cavity, where a part of the lysed septum can be seen. Considering the frequent multiplicity of the cysts, the question of topography arises to provide aspiration or surgical excision; and the ABVS technique can help here. An ABVS tomogram performed in “mammographic” positioning helps to determine the cysts’ topography more accurately than HHUS (Fig. 4.24).


Fig. 4.24
Fibrocystic breast disease in a 51-year-old female. Comparison of HHUS, MMG, and ABVS data. (a) HHUS showed large cystic lesions. (b, c) A side-by-side comparison of the corresponding images of MMG (left part) and ABVS data (right part). Craniocaudal MMG view and R SUP (superior-to-inferior) ABVS view (b), MLo MMG and R LAT (latero-medial) ABVS view (c). MMG clearly shows the dense oval-shaped lesion on the background of dense breast tissue. ABVS differentiates this region as clustered cysts. Septations are clearly seen on R LAT ABVS image (arrow). Localization and the shape of the cysts are the same on MMG and on ABVS in comparable projections. Clustered cysts and dilated ducts (1), preserved glandular tissue (2) in the upper-outer quadrant
Complex atypical cysts can present with indistinct boundaries, with a lack of enhancement behind the cyst, and with the presence of internal echoes due to sediment as a result of inflammation and increased protein content, blood clots, or tumors, and therefore the cyst becomes indistinguishable from a solid mass. ABVS shows a very characteristic pattern of cysts that can distinguish them from cancer. The presence of an area of an unrimmed stamped black “hole” without “retraction” phenomenon within the breast tissue will testify in favor of cysts (Figs. 4.25 and 4.26). However, this diagnosis without a biopsy may not be safe.



Fig. 4.25
A cyst with hemorrhagic content in a 55year-old patient with a fibrocystic change. Comparison of HHUS, MMG, and ABVS data. (a) HHUS. A hypoechoic mass with irregular margins and slight distal shadowing is noted. (b) MMG of the inner quadrant of the left breast. Dense round-shaped cystic type mass (arrow). (c) A side-by-side comparison of the corresponding images of MMG (left part) and ABVS data (right part). Craniocaudal MMG view and L SUP (superior-to-inferior) ABVS view. A “hole-like” area with stamped contours is found in the middle of the echoic glandular tissue on the ABVS tomogram, not distorting the parenchymal pattern, without a capsule and “retraction” pattern, which is typical for cysts

Fig. 4.26
Atypical hemorrhagic cyst in the right breast in a 59-year-old woman with fibrocystic change. Comparison of HHUS, MMG, and ABVS data. (a) HHUS showed a cystic mass with echogenic content. (b) ABVS image analysis on the workstation of the R LAT slices (right latero-medial view). Anechoic structure with smooth contours without distinct rim. (c) Mammograms of the right breast CC and MLo views. Dense round mass in the central part of the right breast. (d) ABVS image in multiplanar reconstruction mode performed on the US device. Cross-sectional view through the cyst
Dyshormonal hyperplasia can cause an increased echogenicity of the breast parenchyma due to fibrosis. An acoustic shadow often appears behind the regions of fibrosis, obscuring differentiation of the underlying structures. Diffuse forms of dyshormonal hyperplasia should be controlled with follow-up and treated to normalize the hormonal level. In 8.5 % of cases, an overlying infection causes perifocal inflammation [30]. The mass in such patients can possess the characteristics of a malignant tumor. Wall thickening, increased vascularization in the walls, disappearance of anechogenicity, and blurring contours can require an additional examination with follow-up and sometimes biopsy.
In the following case, a 29-year-old woman presented with symptomatic painful left breast over a week and an ultrasound exam showed a tender 10*15 mm mass with stellate appearance with thickened walls, internal echoes, peripheral vascularity, and multiple cysts throughout the breast. An ABVS tomogram showed an echo-poor mass in the upper-outer quadrant with slightly distorted contours without “retraction” phenomenon. The case was classified as BI-RADS 4 and anti-inflammatory treatment was prescribed; a follow-up exam and biopsy were recommended. The follow-up study 2 weeks later showed only multiple small cysts within the upper-outer quadrant, without areas of changed vascularization (Fig. 4.27).




Fig. 4.27
Follow-up study of non-lactation mastitis with a complex cyst in a 29-year-old patient. The aspirate consisted of thick material resembling pus. The cytology showed numerous macrophages in an amorphous background. No epithelial cells or malignant cells are seen. Comparative results of HHUS and ABVS data ((a–c) US study before treatment, (d) follow-up study after the treatment). Explanation in the text. (a) Gray scale HHUS. (b) HHUS in color Doppler mapping (CDM). (c, d) ABVS images of the left breast
A stellate pattern can appear sometimes in cysts due to fibrosis or inflammation. This can subsequently also form a spiculation on a mammogram simulating a malignant lesion. ABVS in those cases may show doubtful results which are indistinct from cancer (Figs. 4.28 and 4.29).



Fig. 4.28
A case of a complex cyst with contents which simulate a malignant lesion. Comparison of HHUS and ABVS data. (a) HHUS in Color Doppler mode. A mass with irregular contours, sized up to 10 mm, with visible distal acoustic shadowing and perinodular vascularization. (b) ABVS image obtained in LAT view (latero-medial oblique). An undulating contour simulates “retraction” phenomenon (arrow). The biopsy showed a bloodstained substance with apocrine metaplasia, without atypia

Fig. 4.29
A case of a cyst with previous inflammation in a patient with a fibrocystic change. Histopathology revealed stromal hyalinosis and leukocyte infiltration; no atypical cells were found. (a) ABVS obtained in AP view (anteroposterior). Multislice view through the lesion based on the sagittal slice (right part), perpendicular coronal slice of the same lesion (left part). A spiculation pattern is presented on the coronal view of the lesion corresponding to the zone of postinflammatory changes and cystic rearrangement (arrow), simulating a malignant pattern. (b) ABVS. Multislice view through the lesion based on the axial slice (right part), perpendicular coronal slice of the same lesion (left part). The hypoechoic region with spiculation on the coronal slice is presented in the zone of postinflammatory changes
The frequency of neoplastic changes inside the cysts is not very high. Intracystic papillary breast cancer is a rare pathology. Its frequency ranges from 0.28 to 0.5 % of breast cancer in general and 0.05 % of all cysts [25]. Pneumocystography was replaced by ultrasonography in assessing intracystic papillary masses [32]. Ultrasound accurately visualizes the internal papillary components and differentiates them from debris demonstrating vascularity. Malignant masses have wide basement and indistinct external boundaries. ABVS can also confirm the papillary components inside large cysts if anechoic fluid is present (Fig. 4.30). Multiplanar reconstruction or slice-by-slice previewing through the cyst easily reveals cysts with solid lesions.


Fig. 4.30
Cysts with papillary components in a 53-year-old woman with fibrocystic change. Comparison of HHUS and ABVS data. (a) HHUS. Multiple cysts with a papillary echogenic intraluminal component. (b) ABVS. Multislice view through the lesion (right part), latero-medial oblique slice perpendicular to the sagittal slice (left part). Papillary components are visible inside the cysts not altering the outer contours of the cysts
4.4 Breast Cancer
Breast cancer is common in women and a leading cause of cancer mortality in women worldwide [33]. Early preclinical diagnosis is of significant value because the sooner a tumor is detected, the longer the life expectancy of the patient. Life expectancy in breast cancer is influenced by the tumor size, presence or absence of metastases in regional lymph nodes, presence of receptors to female sex hormones in the tumor, histological type of the tumor, and many other factors. The number of such factors is growing as tumor biology is studied. Breast cancer is a heterogeneous disease with variations in morphological picture, clinical course, and sensitivity to treatment. This is due to the differences in tumor biology at the molecular level. The most aggressive are TN (triple-negative) and HER2-positive carcinomas. The most common histological type is considered to be ductal carcinoma, less common are lobular, mixed, medullary, colloid, nipple, and others. The numerous types of breast tumor cause polymorphism of their clinical, mammographic, and sonographic manifestations. The stage of the tumor is directly related to its size and spread. Therefore, any diagnostic method should not only identify the primary tumor but also assess the extent it has spread.
Therefore, the interest in developing new methods of early and specific diagnosis of non-palpable and “minimal” breast cancers of less than 1 cm is growing. X-ray mammography is established as a “gold standard” for assessing breast structure and breast density and for identifying regional axillary lymph nodes. MMG screening reduces mortality from breast cancer by more than 30 % [34]. However, the sensitivity of mammography depends on the breast density. Studies on women with dense breasts have demonstrated a sensitivity of MMG of less than 50 % [35]. More than 50 % of women younger than 50 and at least one-third aged over 50 have been found to have dense breast tissue [36]. Thus, cancer will be missed mammographically in every second woman with a dense breast. A mammogram is a summation image with all breast tissue overlapping in each view, thus resulting in missed cancer. It was found that the odds of interval cancer among women with extremely dense breasts were 17.8 times greater than among women with fatty breasts. In addition, breast density has been established as an independent risk factor for breast cancer [9].
Breast ultrasound, as currently considered among breast imaging modalities, has an essential and specific role as a complementary method to mammography for woman with dense breasts and clinically or radiologically detected suspicious breast lesions [37]. A combination of mammography with ultrasound in women after 40 with radiographically dense breasts doubles the detection of cancers [38, 39]. HHUS represents the gold standard for examination of young patients in which mammography is not indicated because of exposure of the breast to ionizing radiation and risk of induced breast cancer. However, the dependence on the operator for HHUS is a major concern with respect to the widespread use of whole-breast US.
Automated breast volume imaging has several advantages over HHUS: the technique is more reproducible, has 3D capability with multiplanar reconstruction, and allows delayed interpretation outside real time, optimizing the radiologist’s reading environment [40]. ABVS provides additional information to radiologists about breast cancer, especially in women with dense breast. A coronal view of the entire volume offers an easily understandable representation of the global breast anatomy and architecture, and also helps surgeons, giving a comprehensive sight of the breast from the skin line to the chest wall [23]. Breast cancer, especially IDC, shows a “retraction” phenomenon on ABVS coronal views, which is valuable in differentiating lesions as malignant [41, 42]. The high sensitivity of ABVS in detection of breast cancer was shown in many studies [23, 43–47].
4.4.1 Spiculated Breast Cancers
Cancer has a very typical stellate pattern on a mammogram, with a central dense region with uncircumscribed margins, which extend into the surrounding tissue as several gradually tapering strands. They are formed as a result of desmoplastic reaction around the tumor and periductal fibrosis. The spiculation of the tumor, “retraction” phenomenon, and “radiance” are features of malignancy. These changes are usually visible on X-ray MMG. But sometimes overlapping effect and increased breast density masks this sign. In these cases, additional methods of examination are needed, for example, X-ray digital breast tomosynthesis, which helps to identify a tumor site more accurately by obtaining a series of subsequent breast images. The alternative is ultrasound examination. However, before the era of 3D breast ultrasound, it was not always possible to see this phenomenon using conventional 2D ultrasound. Various artifacts, while they mechanically collected US volumetric data, precluded the full identification and assessment of the significance of the findings in the diagnosis of breast cancer. ABVS technology is able to detect this phenomenon. The dimensions of the tumor and molecular type of the tumor do not affect the severity of the symptoms. The “retraction” symptom is clearly seen even in nodules less than 5 mm [48, 49]. Around the tumor, a hyperechoic halo develops as a crown, with a hypoechoic central zone (Fig. 4.31).
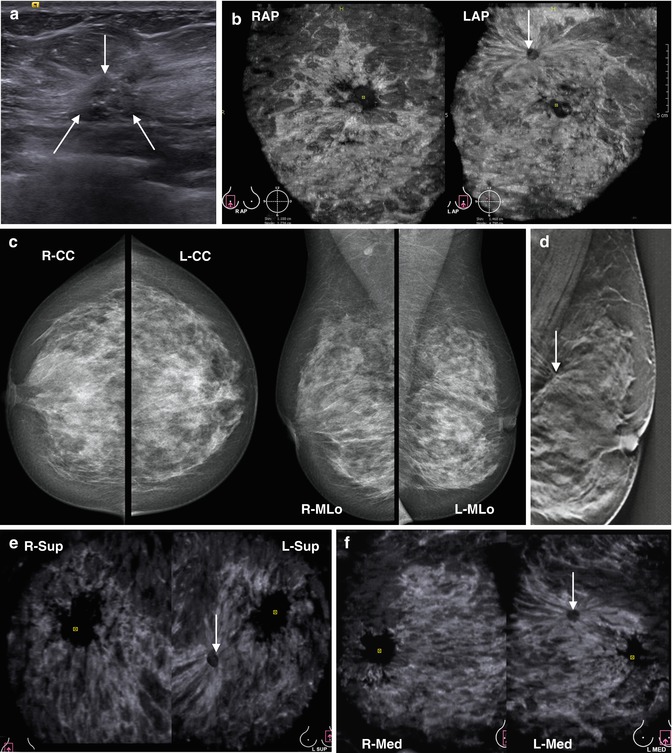

Fig. 4.31
Invasive ductal carcinoma in the upper-inner quadrant of the left breast in a 50-year-old female not found with palpation. Histopathology, immunohistochemistry: T1aN0M0, TN, grade G1. Multimodality study. Comparison of HHUS, ABVS, MMG, DBT findings. (a) Gray scale HHUS shows the tumor nodule less than 1 cm with irregular margins and microcalcifications lying within the hyperechoic breast fibrous tissue (arrows). (b) On bilateral ABVS of right and left breasts, an asymmetry is noted on coronal projections (R AP and L AP). In the upper-inner region of the left breast, a hypoechoic lesion with severe retraction phenomenon is seen (arrow). (c) Mediolateral oblique and craniocaudal bilateral breast mammograms (R-CC and L-CC; R-MLo and L-MLo) show a heterogeneously dense breast with no discernible abnormality. The cancer was missed by MMG due to dense breast and the particular localization of the tumor (upper-inner quadrant). (d) Digital breast tomosynthesis of the left breast in mediolateral oblique projection reveals the subtle tumor nodule with severe spiculation (arrow). (e, f) Bilateral ABVS. Side-by-side comparison of the symmetrical projections (e) R-Sup (left part) and L-Sup (right part); (f) R-Med (left part) and L-Med (right part). The tumor nodule with spiculation is clearly visible on both views (arrow)
The “retraction” phenomenon is characteristic for 90 % of breast cancer cases. A. Broberg et al. (1983) noted that the nodes with spicules usually occur in women with a high content of estrogen receptors in the breast [50, 51]. However, we have not noted any clear relationship of their content with the histological grade of differentiation and molecular type of the tumor. All cases of basal and luminal type A cancer demonstrated this sign, and in luminal type B and Her2neu overexpressing tumors, this sign was observed in 80 % of cases (Fig. 4.32).
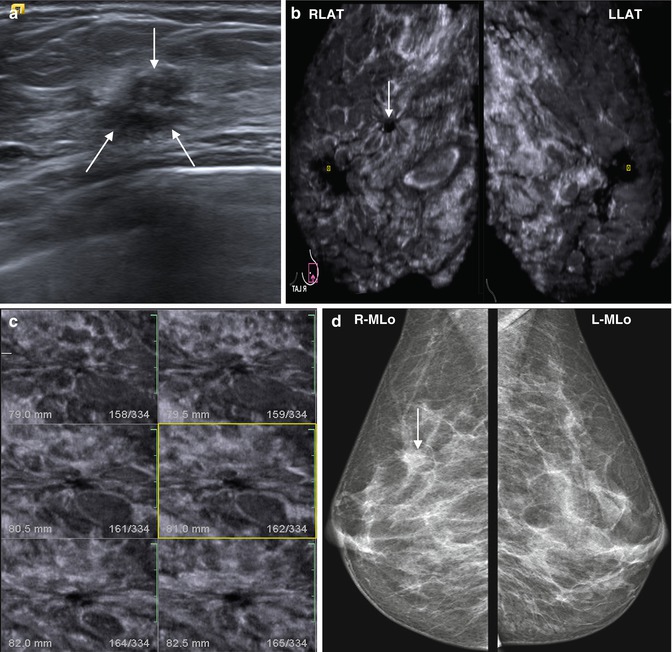

Fig. 4.32




A 44-year-old woman with palpable abnormality in the upper-outer quadrant of the right breast. Histopathology proved IDC, T1aN0M0, luminal type A, grade G2. Comparison of the performance of HHUS, ABVS, MMG. (a) A hypoechoic nodule less than 1 cm with echogenic halo and indistinct boundaries with increased anteroposterior size is seen on HHUS (hyperechoic halo caused by desmoplastic reaction). (b) Bilateral ABVS tomograms. Comparison of R-Med (right mediolateral oblique—left part) and L-Med (left mediolateral oblique—right part) projections. The lesion with spiculation effect is clearly visible in the right gland (arrow). (c) A multislice view with creation of serial sections through the tumor obtained by ABVS. Coronal slices of the tumor with a 1 mm slice thickness. The “retraction” phenomenon is clearly visible. (d) Bilateral MMG. Side-by-side comparison of MLo views (R-MLo and L-MLo). The nodular density in the upper part of the right breast is more prominent within the dense breast (arrow)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree