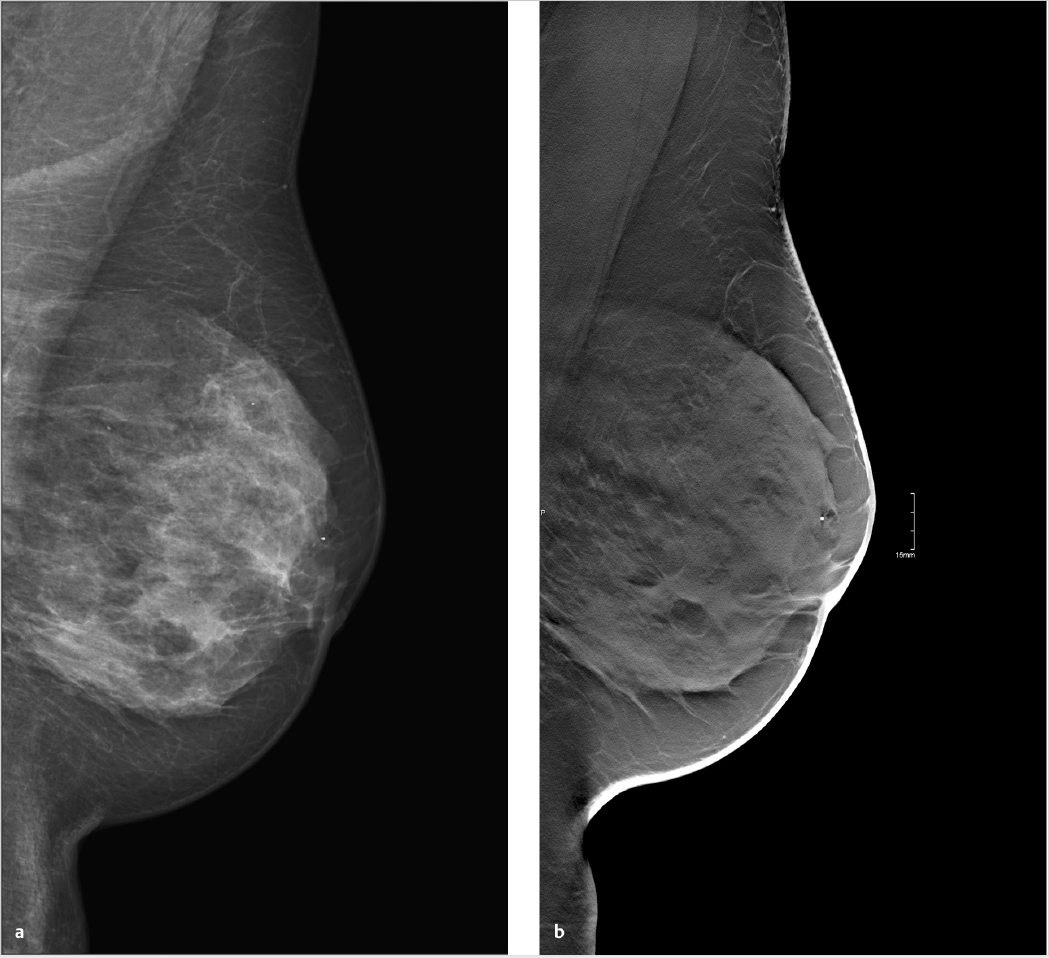
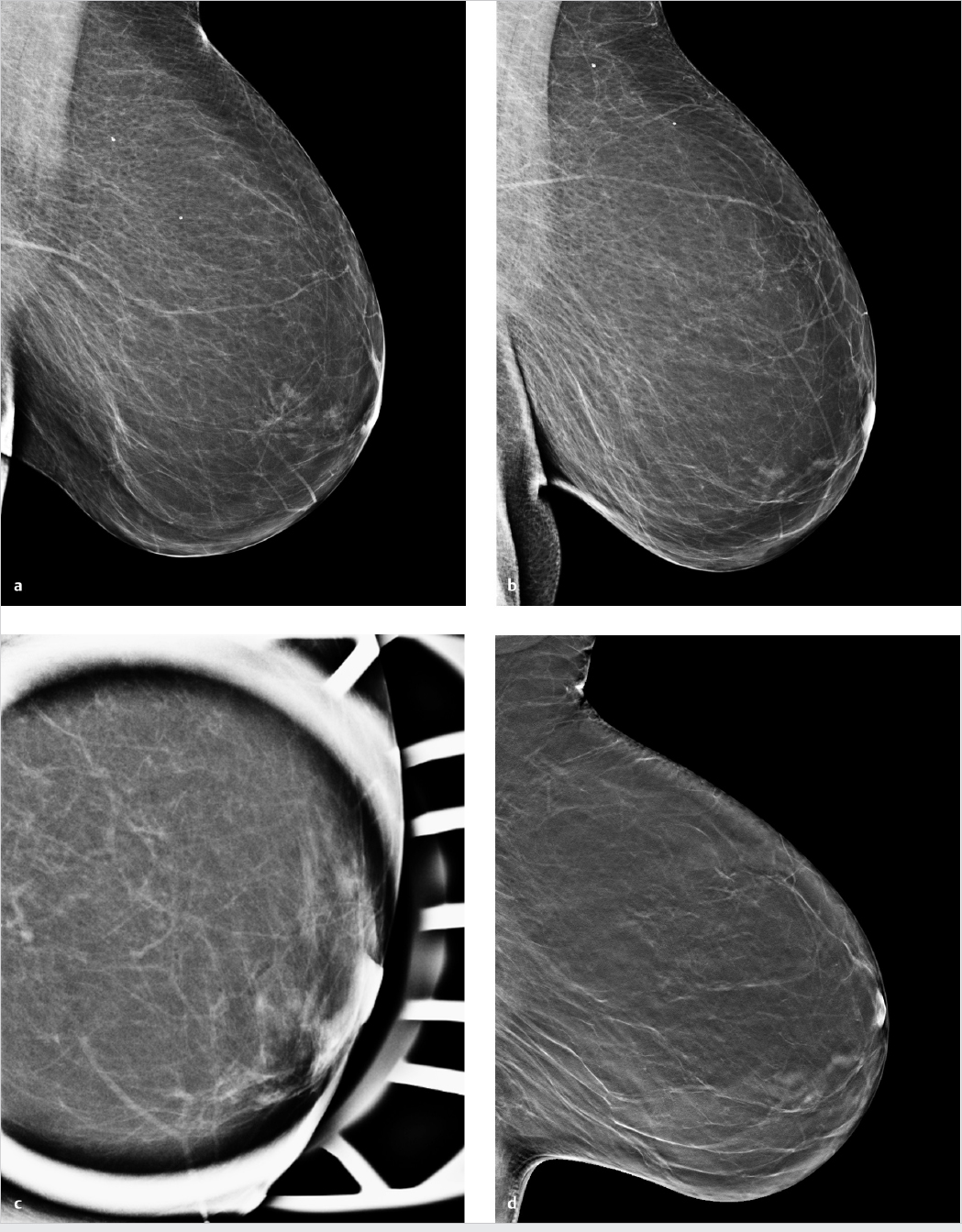
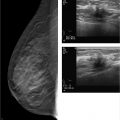
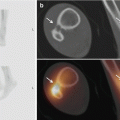
Chapter 3 3.2 Mammography versus Mammography Plus Tomosynthesis 3.3 Tomosynthesis versus Additional Mammographic Views 3.4 Mammography versus Tomosynthesis There is no question that full-field digital mammography continues to be the gold standard for breast imaging. Though it has lower spatial resolution than screen-film mammography, digital technology offers definite advantages in both image acquisition and display and has largely replaced screen-film mammography. The advantages of full-field digital mammography include high quantum efficiency, excellent contrast resolution, and a large dynamic range. Thus, full-field digital mammography provides the necessary diagnostic image quality, while also meeting all requirements in terms of radiation safety.1 However, digital technology alone cannot overcome the inherent limitation of mammography. Since mammography is a projection technique, it yields a summation image in which many anatomic structures with similar absorption characteristics or densities are superimposed. This can hamper detection and characterization of lesions. The sensitivity of mammography is greatly limited in radiographically dense breasts with an American College of Radiology (ACR) rating of 3 or 4. As a result, physiologically dense glandular structures may overlie and obscure suspicious changes such as focal lesions or architectural distortions. Moreover, conventional projection mammograms often yield false-positive findings, owing to summation effects. This occurs when normal breast structures are superimposed in the path of the X-ray beam, creating an overlap density that requires further evaluation by supplementary methods such as breast ultrasonography or special views. Digital breast tomosynthesis (DBT) attempts to add information and overcome the limitations of mammography. The reconstruction of tomographic image slices of the breast eliminates summation effects and improves the detectability of focal lesions. Various questions must be addressed in order to define the clinical role of DBT as accurately as possible and establish guidelines for its use. A number of studies have focused on several key issues: • Does tomosynthesis add information to that supplied by digital mammography? • Can tomosynthesis replace additional mammographic views? • Can tomosynthesis replace mammography? Another issue to be considered is radiation exposure. The ultimate goal is clear: to achieve greater diagnostic accuracy at a lower dose. This underscores the need to achieve higher sensitivity and specificity in DBT than in standard two-view mammography. Progress must be gradual, however, for two reasons. First, the technical evolution of DBT is far from complete; and second, more extensive clinical experience and ongoing training in the evaluation of tomosynthesis are needed in order to fully establish the diagnostic potential of this technology. Various studies have already been done to determine whether tomosynthesis can add information to that provided by digital mammography. In each of these studies, mammography alone was compared with a combination of mammography and tomosynthesis. In an initial clinical study, Poplack et al studied a cohort of 98 women with abnormal digital screening mammograms who required recall.2 In all patients, a total of 11 low-dose exposures were taken in a 19-second scan and were reconstructed into tomosynthesis images. The study confirmed that the rate of recall based on findings detected on screening could have been reduced by 40% by the addition of tomosynthesis. The benefits of tomosynthesis over mammography resulted from the more accurate evaluation of lesion margins and the minimization of summation effects from overlapping tissues. Tomosynthesis improved accuracy in the detection or exclusion of architectural distortions, asymmetries, and focal lesions (masses) (Fig. 3.1). Tomosynthesis did not prove helpful for the morphologic analysis of suspicious microcalcifications in this study. The authors concluded that, for evaluation of calcifications, the image quality from tomosynthesis was inferior to that of full-field digital mammography. This may have resulted from motion artifacts or the low reconstructed slice thickness. A key limitation in this study, however, was the small number of cases. Building on the encouraging initial results, further studies were undertaken in larger groups of patients.3,4 The studies differ primarily in their inclusion criteria, number of projection images acquired, and study design. A large multicenter study with a total of 27 readers compared the diagnostic accuracy of two-view mammography alone or combined with two-view tomosynthesis.5 Two groups were formed from the study population of more than 1,000 women. Study 1 comprised 312 cases with a total of 48 cancers and images read by 12 radiologists. The readers determined only whether an abnormality requiring recall was present or absent. In study 2 (310 cases, 51 cancers, 15 radiologists), the lesion type and location were also recorded. All 27 radiologists found that the addition of tomosynthesis improved diagnostic accuracy, while the recall rates for individual readers were reduced by between 6 and 67%. The addition of tomosynthesis was particularly effective in improving the detection of invasive cancers, whereas it provided little gain in the detection of in situ cancers. Based on their results, the authors concluded that the addition of tomosynthesis to digital mammography improves diagnostic accuracy and significantly reduces the recall rates for noncancer cases. In summary, all studies in patients with suspicious mammograms have shown that tomosynthesis provides a diagnostic gain when used as an adjunct to mammography. It is unclear from the studies whether tomosynthesis could also be used effectively in an unselected population and in an algorithm that includes double reading. This question was addressed in the Oslo Tomosynthesis Screening Trial.6 A total of 12,621 women from a nationwide screening program in Norway underwent bilateral mammography in the craniocaudal (CC) and MLO projections plus bilateral tomosynthesis in the CC and MLO projections. Both the conventional and tomosynthesis images were acquired during a single breast compression per view. Four experienced radiologists read the images in four different study arms: • Two-dimensional (2D) mammography alone. • 2D mammography plus computer-aided detection (CAD). • 2D mammography plus tomosynthesis. • Synthesized 2D images reconstructed from the tomosynthesis data, plus three-dimensional (3D) tomosynthesis. All examinations that were rated suspicious in one of the study arms were subsequently discussed in a consensus meeting and referred for recall if necessary. The addition of tomosynthesis increased the recall rate: 365 women with suspicious mammographic findings were recalled, compared with 463 women with suspicious findings by mammography or tomosynthesis. This led to a 30% increase in the cancer-detection rate in the study arms with adjunctive tomosynthesis. In absolute terms, the cancer-detection rates were 0.71% with mammography alone (7.1 cancers per 1,000 women screened) and 0.94% in the tomosynthesis groups. Most of the additional cancers detected by tomosynthesis were node-negative invasive tumors. The cancer-detection rate relative to the total number of recalls was approximately 25% in both study groups, with or without tomosynthesis. Besides diagnostic accuracy, this study also addressed two other important aspects. The average reading time for 2D mammography was 48 seconds per reading, but this increased to 89 seconds when tomosynthesis was added. This doubling of the reading time would significantly increase the workload in a screening program. The second aspect is radiation exposure. The average parenchymal dose was 1.58 ± 0.61 milligray (mGy) for mammography, 1.95 ± 0.58 mGy for tomosynthesis, and 3.52 ± 1.08 mGy for the combination of mammography and tomosynthesis. Another study was conducted in a total screening population of 13,158 women.7 All patients underwent two-view digital mammography, and 6,100 of the women underwent digital mammography plus two-view DBT. The cancer-detection rates in the two groups were compared. In contrast to the study by Skaane et al,6 the recall rate in the DBT group, at 8.5%, was lower than in patients receiving mammography alone (12%). The cancer-detection rate was 0.52% with mammography alone versus 0.57% with mammography plus tomosynthesis (P= 0.70). Recently, Friedewald et al8 published a retrospective analysis of screening performance metrics from 13 sites in the United States of America, using mixed models adjusting for site as a random effect. A total of 281,187 digital mammographies and 173,663 combined examinations (digital mammography plus tomosynthesis) were evaluated. Model-adjusted rates per 1,000 screens resulted in a recall rate of 107 with digital mammography versus 91 for digital mammography plus tomosynthesis. The number of biopsies increased by 1.3 per 1,000 screens (18.1 with digital mammography versus 19.3 with digital mammography plus tomosynthesis). Cancer detection significantly increased from 0.42% with digital mammography to 0.54% for digital mammography plus tomosynthesis. This large multicenter study confirmed the results of the previous studies and demonstrated that the addition of tomosynthesis to digital mammography resulted in a decreased recall rate and an increased cancer-detection rate. In summary, all the studies confirm that in both selected and screened populations, the addition of tomosynthesis to digital mammography led to improved diagnostic accuracy and an increase in cancer-detection rates. A number of questions remain to be answered, however. In particular, data are needed that can show whether tomosynthesis actually requires two views when used as an adjunct to mammography. Because tomosynthesis supplies 3D information, one-view tomosynthesis may be sufficient. Further large studies are needed to resolve this issue. The routine combination of two-view mammograms with one- or two-view tomosynthesis increases diagnostic accuracy but also raises concerns about radiation safety. It is common in routine clinical settings, however, for two-view mammograms to yield equivocal results that require further evaluation by additional views (e.g., spot compression and microfocus magnification views). This raises the question of whether tomosynthesis could be useful in these cases and whether it is equivalent or even superior to additional mammographic views (Fig. 3.2). In 2010, Hakim and his group compared the value of adjunctive digital tomosynthesis with additional mammographic views in the characterization of known mammographic masses, architectural distortions, and asymmetries.9 Four experienced radiologists interpreted full-field digital mammograms aided by additional views or DBT images, in 25 women. The radiologists were asked to determine which combination yielded the best diagnostic accuracy. In 50% of the ratings, the radiologists perceived the combination of full-field digital mammography plus DBT to be diagnostically superior to full-field digital mammography plus additional views. Both combinations were rated equivalent in 31% of cases, and the addition of DBT was rated as inferior to additional mammographic views in 19%. Over all the readers, 92% of the ratings for known breast cancer cases and 50% of the ratings for known high-risk lesions were classified as BI-RADS 4 or 5. The authors concluded that DBT provided an alternative to additional mammographic views for the characterization of focal abnormalities detected in two-view mammograms.
Clinical Evaluation of Digital Breast Tomosynthesis
3.1 Introduction
3.2 Mammography versus Mammography Plus Tomosynthesis
3.3 Tomosynthesis versus Additional Mammographic Views
Radiology Key
Fastest Radiology Insight Engine