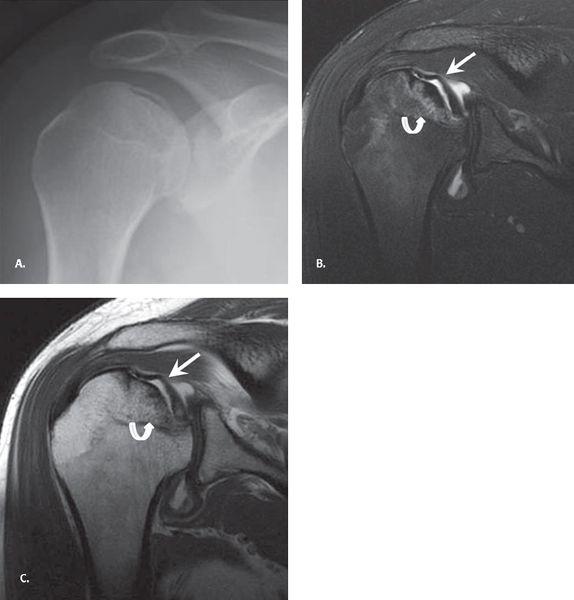
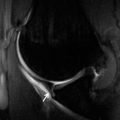
FIGURE 7.21 Ankylosing spondylitis. Anteroposterior plain radiograph of the shoulder demonstrating irregular bony proliferation about the erosions and at tendon and capsular attachments (arrow).
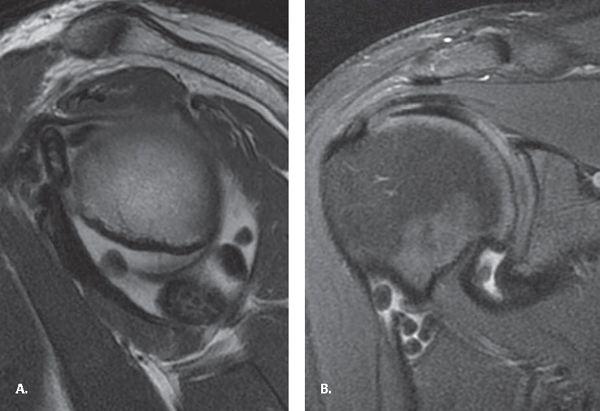
FIGURE 7.22 Synovial osteochondromatosis. A: Sagittal proton density fast spin-echo (PD FSE) and (B) coronal fat-suppressed PD FSE images demonstrating multiple uniformly sized nodules throughout the glenohumeral joint, which appear predominantly as dark signal intensity resulting from calcification. Fat is seen within the largest nodule resulting from marrow formation.
Synovial Osteochondromatosis
The primary form of this benign monoarticular arthropathy results from cartilaginous metaplasia of the synovium. The knee is the most commonly affected joint followed by the elbow, hip, and shoulder. The classic description of imaging findings is multiple uniformly sized nodules throughout the joint, but in actuality, nodules can vary in size. The nodules demonstrate variable mineralization with no calcification seen in up to 30% of cases. CT scan is more sensitive when there are only faint calcifications. Calcified bodies appear dark on both T1- and T2-weighted images on MRI and can mimic PVNS. MRI, however, can be used to confirm the presence of intra-articular nodules and demonstrate the T2 hyperintensity characteristic of cartilaginous lesions. Another imaging feature on MR is fat within the nodules resulting from fatty marrow formation from ossification of the nodules (Fig. 7.22).
Amyloid Arthropathy
The most common cause of systemic amyloidosis is B2 microglobulin deposition in patients on long-term hemodialysis. The shoulder is the most common symptomatic joint followed by the hip, knee, and wrist. Oligoarticular involvement is common. Radiographic findings are non-specific and can include periarticular erosions, well-defined bone lesions (amyloidomas), and late joint space loss. The bone lesions are common in the shoulder and hip and can be confused with the brown tumors of hyperparathyroidism also seen in patients on long-term hemodialysis. Secondary hyperparathyroidism also features subperiosteal resorption on radiographs and can be diagnosed by blood chemistry. The diagnosis of amyloidosis, however, requires synovial biopsy.
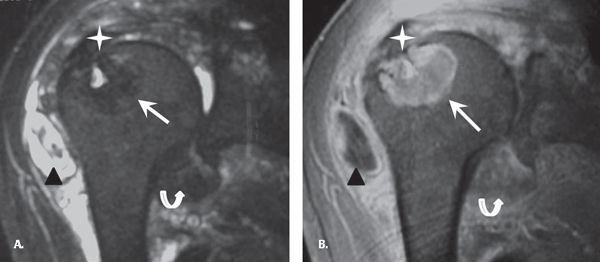
MRI findings in the shoulder include thickening and decreased T2 signal of the rotator cuff tendons and glenohumeral joint capsule. Intra-articular nodular lesions demonstrate decreased T2 signal intensity and minimal peripheral enhancement. The bone lesions demonstrate variable T2 signal and moderate enhancement. This variable T2 signal likely relates to varied amounts of amyloid and fluid within the lesion. The subacromial and subdeltoid bursa can appear irregular and thickened with loculated bursal fluid collections. The glenohumeral joint can have a large joint effusion and there may be focal fluid within the biceps tendon sheath (41). The differential considerations for MR findings include PVNS, hemophilia, and hemorrhagic geodes (Fig. 7.23).
Neuropathic Osteoarthropathy
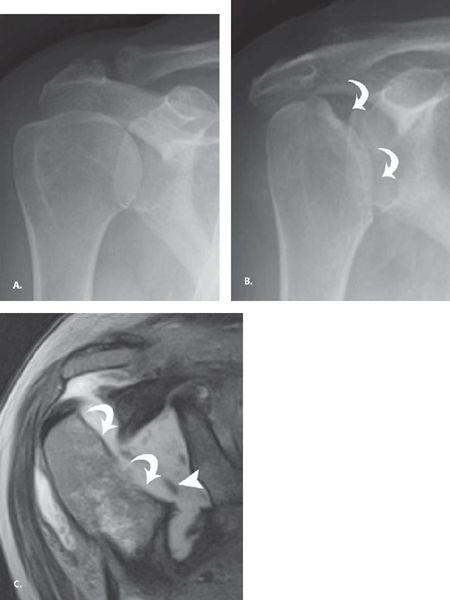
The most common cause of neuropathic shoulder is syringomyelia (greater than 75%) with other causes, including diabetes, trauma, peripheral neuropathy, and spinal tumors. Identification of a neuropathic shoulder should prompt MRI of the cervical spine to look for a syrinx. Both hypertrophic and atrophic patterns occur for neuropathic joints, with nonweightbearing joints like the shoulder demonstrating primarily the atrophic form. The atrophic neuropathic shoulder arthropathy can be rapidly progressive and mimic septic arthritis, Gorham’s vanishing bone disease, and malignancy. Dissolution of the humeral head with sharp margins at the leading edge of resorption is a characteristic finding and unlike the irregular ill-defined resorption seen in septic arthritis (Fig. 7.24). Patients often present with mass-like enlargement of the shoulder resulting from large joint effusion and adjacent fluid collections. Osseous debris is commonly seen and can be mistaken for tumor matrix. However, unlike neoplastic processes, neuropathic disease commonly involves the glenoid side of the articulation as well.
Gouty Arthropathy
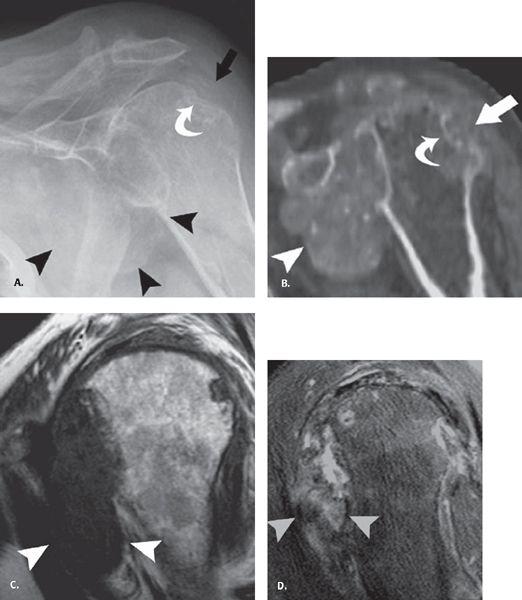
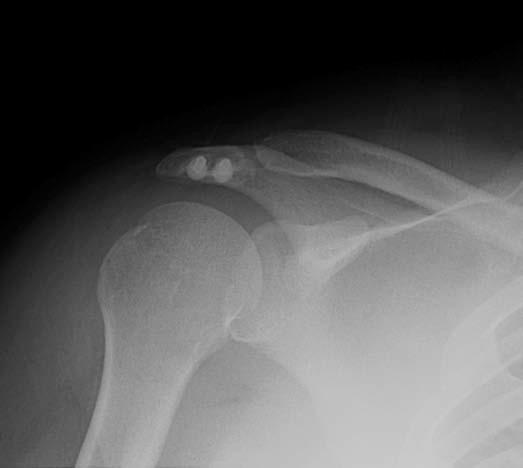
Gouty arthropathy is a polyarticular peripheral arthritis resulting from intra-articular deposition of sodium urate crystals. The most common sites of involvement are the feet, hands, wrists, elbows, and knees, and the shoulder is a rare site. Radiographic findings include mild dense soft tissue mass with well-defined marginal erosions and adjacent overhanging edge of bone (Fig. 7.25). There is usually preservation of joint space until late in the disease and normal bony mineralization. CT attenuation of the gouty tophi is approximately 170 ± 30 Hounsfield units (HU), and presence of apatite crystals can raise the central CT attenuation to 450 HU. Gout has a nonspecific MR appearance. Tophi has variable signal intensity on MR, most commonly intermediate to low on T2 and isointense to muscle on T1. Soft tissue inflammatory change around the low T2 signal intensity mass suggests active tophus. Gout should also be considered in any heterogeneous intermediate to low T2 signal-intensity mass eroding into adjacent bone (42).
FIGURE 7.23 Amyloidosis. A: Coronal fat-suppressed (FS) T2 and (B) fat-suppressed T1 post-intravenous gadolinium images demonstrate thickening and decreased T2 signal of the rotator cuff tendons (star) and glenohumeral joint capsule (curved arrow); predominantly T2 dark intraosseous lesion with small focal T2 hyperintensity likely relates to varied amount of amyloid and fluid within the lesion (arrow). Subacromial and subdeltoid bursa appears irregular and thickened with loculated bursal fluid collections (black triangle). Courtesy Dr. Lynne S. Steinbach.
FIGURE 7.24 Atrophic neuropathic shoulder. A: Frontal radiograph at presentation and (B) frontal radiograph at 6 months follow up show the development of resorption of the humeral head (curved arrow) with a paucity of findings at the glenoid. C: Coronal proton density fast spin-echo image verifies the humeral head resorption (curved arrows) as well as intra-articular debris (arrowhead). Reproduced with permission from Farid N, Bruce D, Chung CB. Miscellaneous conditions of the shoulder: anatomical, clinical, and pictorial review emphasizing potential pitfalls in imaging diagnosis. Eur J Radiol. 2008;68:88–105.
Avascular Necrosis
Avascular necrosis (AVN) may occur with three- or four-part fractures of the proximal humerus with injury to a branch of the anterior humeral circumflex artery and disruption of the blood supply to the humeral head. Nontraumatic etiologies of AVN include idiopathic, steroid use, alcoholism, pancreatitis, and systemic conditions such as vasculitis, sickle cell disease, hyperuricemia, Gaucher disease, familial hyperlipidemia, and lymphoma.
FIGURE 7.25 Gout. A: Frontal radiograph of the shoulder shows erosive change at the greater tuberosity (curved arrow) with dense tophus formation adjacent to it (straight arrow) as well as in the axillary pouch (arrowheads). B: Sagittal reconstructed computed tomographic image confirms these findings. C: Sagittal T1 and (D) fat-suppressed proton density fast spin-echo images profile the axillary pouch tophus and show the low signal on T1 and intermediate to low signal on the longer TE sequence that is characteristic of tophus formation. Reproduced with permission from Farid N, Bruce D, Chung CB. Miscellaneous conditions of the shoulder: anatomical, clinical, and pictorial review emphasizing potential pitfalls in imaging diagnosis. Eur J Radiol. 2008;68:88–105.
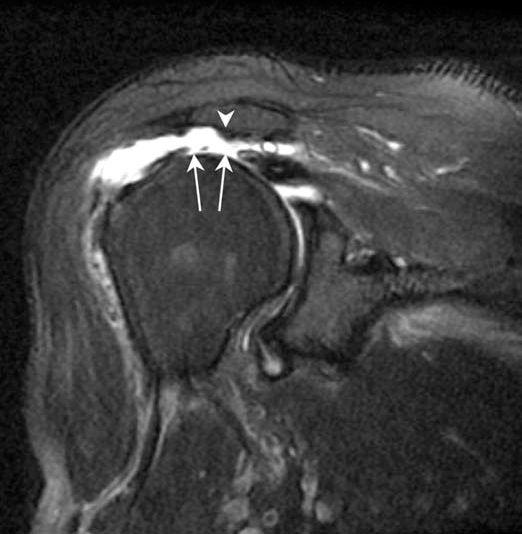
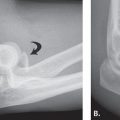
FIGURE 7.26 Avascular necrosis (AVN). A: Anteroposterior plain radiograph demonstrating subchondral lucency and fracture as evidenced by the crescent sign (arrow). B: Coronal fat-suppressed proton density fast spin-echo (PD FSE) and (C) coronal PD FSE images showing subchondral fracture with the crescent sign (arrow) and the double-line sign of a hyperintense inner border and a hypointense peripheral border.
Radiographically, AVN appears normal in the early stage of the disease (stage I) according to Steinberg modification of the classification described by Arlet et al. (43). Subsequently, there may be cystic and/or sclerotic changes of the subchondral bone (stage II) and subchondral lucency and fracture as evidenced by the crescent sign (stage III). In advanced stages, there is subchondral collapse with flattening of the femoral head (stage IV) and secondary arthritis (stage V). On MRI, AVN demonstrates a serpiginous pattern of involvement with a double-line sign (a hyperintense inner border and a hypointense peripheral border on T2-weighted images) and marrow edema (Fig. 7.26) (44).
The Neer (45) classification for AVN of the humeral head is divided into four stages. In stage 1 disease, the patient is asymptomatic, conventional radiograph is normal, and MRI demonstrates subchondral bone marrow edema. Stage 2 disease is characterized clinically by pain. On imaging, the humeral head retains its normal shape; however, mild depression of the articular cartilage and subchondral bone may be present. In stage 3, there is subchondral collapse or fracture with overlying articular cartilage irregularity but without secondary arthritic change. When the disease progresses to stage 4, there are secondary arthritic changes of the glenohumeral joint.
References
1. Gordon BH, Chew FS. Isolated acromioclavicular joint pathology in the symptomatic shoulder on magnetic resonance imaging: a pictorial essay. J Comput Assist Tomogr. 2004;28: 215–222.
2. Bulcholz RW. Rockwood and Green’s Fractures in Adults. Philadelphia, Pa: Lippincott Williams & Wilkins; 2001.
3. Calvo E, Lopez-Franco M, Arribas IM. Clinical and radiologic outcomes of surgical and conservative treatment of type III acromioclavicular joint injury. J Shoulder Elbow Surg. 2006; 15:300–305.
4. Antonio GE, Cho JH, Chung CB, et al. Pictorial essay MR imaging appearance and classification of acromioclavicular joint injury. AJR Am J Roentgenol. 2003;180:1103–1110.
5. Pecci M, Kreher JB. Clavicle fractures. Am Fam Physician. 2008;77:65–70.
6. Neer CS. Fractures of the distal third of the clavicle. Clin Orthop Relat Res. 1968;58:43–50.
7. Peyron JG. Epidemiologic and etiologic approach of osteoarthritis. Semin Arthritis Rheum. 1979;8:288–306.
8. Bonsell S, Pearsall AW 4th, Heitman RJ, et al. The relationship of age, gender, and degenerative changes observed on radiographs of the shoulder in asymptomatic individuals. J Bone Joint Surg Br. 2000;82:1135–1139.
9. De Abreu MR, Chung CB, Wesselly M, et al. Acromioclavicular joint osteoarthritis: comparison of findings derived from MR imaging and conventional radiography. Clin Imaging. 2005;29:273–277.
10. Henry MH, Liu SH, Loffredo AJ. Arthroscopic management of the acromioclavicular joint disorder, a review. Clin Orthop Relat Res. 1995;316:276–283.
11. Tucker TJ, Snyder SJ. The keeled acromion: an aggressive acromial variant—a series of 20 patients with associated rotator cuff tears. Arthroscopy. 2004;20:744–753.
12. De la Puente R, Boutin RD, Theodorou DJ, et al. Post-traumatic and stress-induced osteolysis of the distal clavicle: MR imaging findings in 17 patients. Skeletal Radiol. 1999;28:202–208.
13. Levine AH, Pais MJ, Schwartz EE. Posttraumatic osteolysis of the distal clavicle with emphasis on early radiologic changes. AJR Am J Roentgenol. 1976;127:781–784.
14. Flatow EL, Cordasco FA, Bigliani LU. Arthroscopic resection of the outer end of the clavicle from a superior approach: a critical, quantitative, radiographic assessment of bone removal. Arthroscopy. 1992;8:55–64.
15. Sammarco VJ. Os acromiale: frequency, anatomy, and clinical implications. J Bone Joint Surg Am. 2000;82:394–400.
16. Warner JJ, Beim GM, Higgins L. The treatment of symptomatic os acromiale. J Bone Joint Surg Am. 1998;80:1320–1326.
17. Hayes CW, Conway WF. Calcium hydroxyapatite deposition disease. Radiographics. 1990;10:1031–1048.
18. Resnick D. Diagnosis of Bone and Joint Disorder. 4th ed. Philadelphia, Pa: WB Saunders; 2002.
19. Garcia GM, McCord GC, Kumar R. Hydroxyapatite crystal deposition disease. Semin Musculoskeletal Radiol. 2003;7:187–193.
20. Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis, and management. J Am Acad Orthop Surg. 1997;5:183–191.
21. Moseley HF. Shoulder lesions. Baltimore, MD: Williams & Wilkins, 1969.
22. Hayes CW, Rosenthal DI, Plata MJ, et al. Calcific tendinitis in unusual sites associated with cortical bone erosion. AJR Am J Roentgenol. 1987;149:967–970.
23. Flemming DJ, Murphey MD, Shekitka KM, et al. Osseous involvement in calcific tendinitis: a retrospective review of 50 cases. AJR Am J Roentgenol. 2003;181:965–972.
24. Dieppe PA. Milwaukee shoulder. Br Med J (Clin Res Ed). 1981;283:1488–1489.
25. Steinbach LS, Resnick D. Calcium pyrophosphate dehydrate crystal deposition disease revisited. Radiology. 1996;200:1–9.
26. Stoller DW. Magnetic Resonance Imaging in Orthopaedics and Sports Medicine. 3rd ed. Baltimore, Md: Lippincott Williams & Wilkins; 2007.
27. Bigoni BJ, Chung CB. MR imaging of the rotator cuff interval. Radiol Clin N Am. 2006;44:525–536.
28. Jung JY, Jee WH, Chun HJ, et al. Adhesive capsulitis of the shoulder: evaluation with MR arthrography. Eur Radiol. 2006; 16:791–796.
29. Nakagawa Y, Hyakuna K, Otani S, et al. Epidemiologic study of glenohumeral osteoarthritis with plain radiography. J Shoulder Elbow Surg. 1999;8:580–584.
30. Kerr R, Resnick D, Pineda C, et al. Osteoarthritis of the glenohumeral joint: a radiologic–pathologic study. AJR Am J Roentgenol. 1985;144:967–972.
31. Godeneche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11:11–18.
32. Neer CS II, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65:1232–144.
33. Visotsky JL, Basamania C, Seebauer L, et al. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35–40.
34. Randelli M, Gambrioli PL. Glenohumeral osteometry by computed tomography in normal and unstable shoulders. Clin Orthop Relat Res. 1986;208:151–156.
35. Walch G, Badet R, Boulahia A, et al. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14:756–760.
36. Frankle M, Siegal S, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87:1697–1705.
37. Hong SH, Kim SM, Ahn JM, et al. Tuberculous versus pyogenic arthritis: MR imaging evaluation. Radiology. 2001;218:848–853.
38. Jeon IH, Choi CH, Seo JS, et al. Arthroscopic management of septic arthritis of the shoulder joint. J Bone Joint Surg Am. 2006;88:1802–1806.
39. Chen AL, Joseph TN, Zuckerman JD. Rheumatoid arthritis of the shoulder. J Am Acad Orthop Surg. 2003;11:12–24.
40. Cotton A, Flipo RM, Mestdahg H, et al. Diffuse pigmented villonodular synovitis of the shoulder. Skeletal Radiol. 1995;24:311–313.
41. Kiss E, Keusch G, Zanetti M, et al. Dialysis-related amyloidosis revisited. AJR Am J Roentgenol. 2005;185:1460–1467.
42. Yu JS, Chung C, Recht M, et al. MR imaging of tophaceous gout. AJR Am J Roentgenol. 1997;168:523–527.
43. Steinberg ME, Brighton CT, Hayken GD, et al. Early results in the treatment of avascular necrosis of the femoral head and electrical stimulation. Orthop Clin North Am. 1984;15: 163–175.
44. Mitchell DG, Rao VM, Dalinka MK, et al. Femoral head avascular necrosis: correlation of MR imaging, radiographic staging, radionuclide imaging, and clinical findings. Radiology. 1987; 162:709–715.
45. Neer CS. Shoulder Reconstruction. Philadelphia, Pa: WB Saunders; 1990:363.
Images of the shoulder after surgery can be difficult to interpret. There are a multitude of procedures that are performed and the patient, or even the referring doctor, may not be familiar with the specific procedure that was undertaken. A patient returning with recurrent pain may have a complication of the procedure, from breakdown of repair, degeneration, or a new injury. It is important for the radiologist to assist the referring clinician in delineating the cause. To do this, one must be familiar with advantages and disadvantages of available modalities as well as the basic types of procedures, their imaging characteristics, and potential complications. This Chapter discusses procedures that are commonly encountered.
Use of Imaging Modalities
Radiographs
Radiographs should precede any advanced imaging after shoulder surgery. Baseline anteroposterior (internal and external rotation) and outlet (or “Y”) views are routinely used as a baseline after surgery. An axillary view and Grashey view (scapular anteroposterior, oriented tangential to the glenohumeral joint) are commonly added. Loosening of components can be seen as frank displacement or more subtle lucency around the implant indicative of motion. If advanced imaging is required, surgical history and radiographs can be used to estimate whether significant artifact is anticipated, thus helping determine whether ultrasound, computed tomography (CT), or MRI is best (Table 8-1). Conventional arthrography may be useful for evaluation of prostheses or intra-articular implants suspicious for loosening; contrast extending around the implant is indicative of pathology. It should be noted that communication through the repaired rotator cuff is not necessarily pathologic, so this finding on a conventional arthrogram may be clinically inconsequential. However, arthrography combined with cross-sectional imaging (CT or MRI) offers more comprehensive analysis of the anatomy corresponding to contrast communication and can be extremely useful in the postoperative setting.
Magnetic Resonance Imaging
MRI has been used increasingly for evaluation of the postoperative shoulder because it can provide a global assessment, evaluating the numerous potential causes of recurrent or new symptoms after surgery. However, interpretation is made more challenging by variable degrees of susceptibility artifact. Artifact on MRI is related to a number of factors, including metal type, size, and surface complexity. In general, the more complex the metallic surface, the more artifact is seen. Type of metal also has an effect with titanium causing relatively little artifact and stainless steel and cobalt chrome creating a more severe effect. To a certain extent, the effect on MRI can be predicted, although burring (like with acromioplasty) leaves behind microscopic metal fragments with very complex surfaces, invisible on radiographs, which can leave the MR examination almost uninterpretable. Metal anchors cause artifact but are small enough such that the protocol usually does not need to be altered. Radiolucent bioabsorbable implants, commonly used in current practice, cause little to no artifact on MRI. If artifact is problematic, there are protocol changes that can be useful (1). This includes avoiding use of gradient-echo sequences that enhance artifact resulting from T2* susceptibility effects. Use of spin-echo sequences with a relatively lower TE, higher bandwidth, larger field of view, and higher matrix can significantly reduce metal artifact compared with a standard protocol. In general, fat suppression using selective fat presaturation is prone to accentuation of artifact if there is significant metal present resulting from field inhomogeneity, and simply removing fat suppression can improve image quality. Short tau inversion recovery (STIR) is a good alternative for obtaining fluid-sensitive information with fat suppression. Also, if artifact from an implant is asymmetrically shaped, simply swapping phase and frequency can often recover adjacent tissue signal (Table 8-2).
Direct Magnetic Resonance Arthrography
Although noncontrast MRI has been shown to be accurate in diagnosing recurrent pathology in the postoperative shoulder (2), experience at our institution with direct MR arthrography (MRA) has shown that it can provide improved diagnostic capability over noncontrast MRI and it improves reader confidence and reduces the radiologist’s learning curve (3–5). One of the advantages of direct MRA is that by injecting contrast into the joint, there is distention of the joint capsule and separation of the capsular structures from the labrum allowing for better visualization. Additionally, fluid is forced through or around pathologic lesions in the labrum or rotator cuff allowing for improved sensitivity in detecting these lesions. Another advantage of direct MRA is that it has a higher signal-to-noise ratio compared with noncontrast MRI resulting from the use of T1 weighting, which has less noise compared with T2 or STIR techniques. Higher SNR with direct MRA provides improved contrast resolution, which helps define small structures. The appropriate volume of contrast that should be injected depends on the type of surgery that was performed. For labral/capsular tears, the volume of injection should be kept low (usually 12 mL is adequate) to avoid overdistention, which increases the chance of extracapsular leakage. For rotator cuff repair/impingement surgery, the volume of injection should be maximized for the purpose of distending the joint capsule to its end point and forcing contrast through defects in the cuff. Injection is performed until capsular resistance is reached or until pain prevents full distention, which typically occurs after injection of 16 mL if there is no cuff defect. Note should be made that postoperative scarring of the subacromial/subdeltoid bursa can prevent contrast extension. A 2- to 3-mmol solution of gadolinium contrast is optimal; this can be prepared in a 1:200 dilution with saline (i.e., 1 mL of gadolinium in 200 mL of saline). If there is a delay expected before imaging is to be performed, the syringe can be rinsed with 1:1000 epinephrine before the procedure before the gadolinium is drawn up to constrict synovial vessels and reduce the rate of joint fluid resorption.
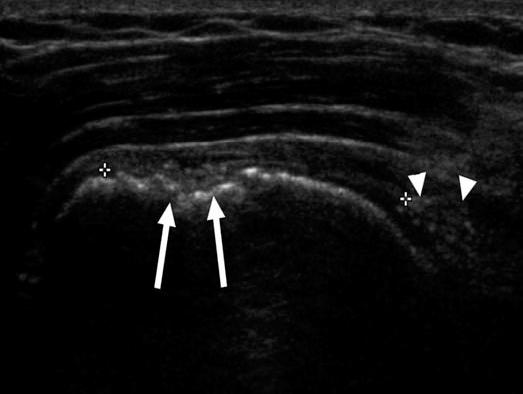
FIGURE 8.1 Coronal ultrasound image of the shoulder of a patient after rotator cuff repair shows depressions (arrows) in the surface of the greater tuberosity representing sites of suture anchor placement. Note large retear of the supraspinatus with retraction (arrowheads).
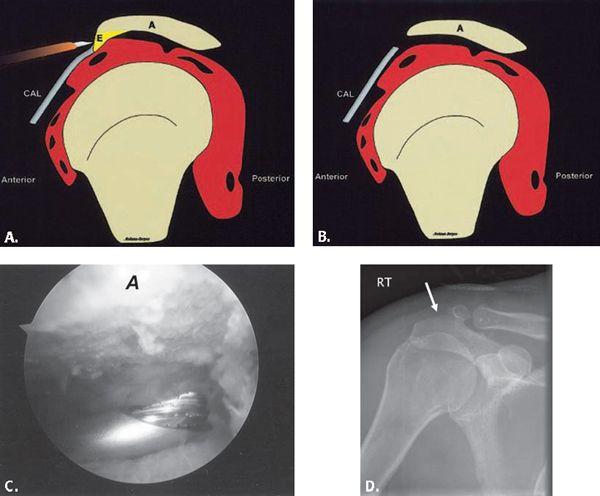
FIGURE 8.2 A–B: Diagrammatic representation of an acromioplasty procedure. The subacromial spur is resected and the undersurface of the acromion is shaved with a burr so that no bony prominence remains. An anterior approach is used for open procedures and a posterior approach is used for the arthroscopic approach. A, acromion; CAL, coracoacromial ligament; E, enthesophyte. (From Mohana-Borges AVR, Chung CB, Resnick D. MR imaging and MR arthrography of the postoperative shoulder: spectrum of normal and abnormal findings. Radiographics. 2004;24:69–85 [37] with permission.) C: Arthroscopic image shows burr instrument shaving acromial undersurface. (Image courtesy of Joseph Abboud, MD, Philadelphia, Pa.) D: Frontal radiograph of the shoulder with deficiency of the anterolateral acromion (arrow) representing prior acromioplasty.
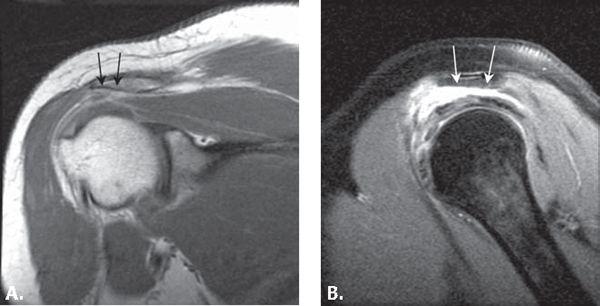
FIGURE 8.3 Expected MRI appearance after acromioplasty. Coronal T1-weighted (A) and sagittal T1-weighted fat-suppressed (B) MR images after intravenous contrast injection. The undersurface of the acromion (arrows) after acromioplasty optimally is flat without residual point or angle. In this case, the coracoacromial ligament is not visualized and has presumably been lysed.
Indirect Magnetic Resonance Arthrography
Indirect MRA is performed by intravenous administration of the standard dose of gadolinium (0.1 mmol/kg) followed by delayed MRI (after approximately 30 minutes). The intravenous contrast is taken up into the joint fluid, providing an arthrographic effect. However, there is no distention of the joint capsule (unless the patient already had a joint effusion), which may reduce the sensitivity for detecting cuff or labral pathology. Signal–to-noise ratio is also lower with indirect MRA and postoperative granulation tissue may enhance, which makes evaluation of the surgical site difficult. Despite its limitations, indirect MRA can be a useful alternative if the patient refuses intra-articular injection or if logistic considerations prevent performance of direct MRA.
FIGURE 8.4 Failed acromioplasty. Coronal T2-weighted fat-suppressed image demonstrating tear of the supraspinatus tendon (arrows) after acromioplasty. Note the osseous prominence of the acromion undersurface (arrowhead), which may have contributed to the tear.
Multidetector Computed Tomography
CT is widely available and relatively low-cost compared with MRI. It is also very useful for evaluation of the postoperative shoulder after certain procedures. Noncontrast multidetector CT (MDCT) yields high-quality images in any plane through isotropic data sets and reformatting capability, even in the presence of large metal implants (6). Consequently, for evaluation of complications (loosening, displacement, fracture) associated with intramedullary rods, cortical plates, screws, and large anchors, MDCT is optimal. Metal streak artifact is minimized by using a higher voltage (140 kVp), higher exposure (200–400 mAs), and reduced pitch with slice overlap (less than 1). These measures result in higher photon flux through the patient and improve image quality; however, these steps also result in increased radiation dose.
Computed Tomography Arthrography
MDCT can be combined with administration of intraarticular iodinated contrast (full-strength is typically used) to achieve a CT arthrogram. This is an excellent technique for detection of labral or rotator cuff retear, especially when significant metal is present. However, it should be recognized that as a result of low tissue contrast, diagnoses can only be made when intra-articular contrast bathes the area of concern. For example, a partial-thickness rotator cuff retear on the bursal side will not be detected on a CT arthrogram. Also, contrast fills in defects but will not detect pathologic scar tissue that contrast does not penetrate.
Ultrasound
Ultrasound can be very useful in some situations (7). It is widely available but demands requisite expertise and proper equipment (e.g., high-frequency probes). Also, patients with limited range of motion may not tolerate positioning required to optimally evaluate the tendons. Nevertheless, because anchors are set within bone and create no artifact, ultrasound is an attractive option for diagnosing rotator cuff retear. In fact, anchors may be visible only as small depressions at the greater tuberosity and can even be overlooked, appearing similar to herniation pits and cortical irregularity commonly present (Fig. 8.1). A corollary of this is that displaced implants (indicating breakdown of repair) are easily detected. On the other hand, ultrasound is disadvantageous for evaluating labral repair and most intra-articular (as well as intraosseous) pathology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree