Neuroimaging plays an essential role in the initial diagnosis and continued surveillance of intracranial neoplasms. The advent of perfusion techniques with computed tomography and MR imaging have proven useful in neuro-oncology, offering enhanced approaches for tumor grading, guiding stereotactic biopsies, and monitoring treatment efficacy. Perfusion imaging can help to identify treatment-related processes, such as radiation necrosis, pseudoprogression, and pseudoregression, and can help to inform treatment-related decision making. Perfusion imaging is useful to differentiate between tumor types and between tumor and nonneoplastic conditions. This article reviews the clinical relevance and implications of perfusion imaging in neuro-oncology and highlights promising perfusion biomarkers.
Key points
- •
Advanced perfusion imaging can offer enhanced evaluation of tumoral physiology to guide the diagnosis, intraoperative sampling, and grading of brain tumors.
- •
Perfusion imaging is useful when differentiating between tumor types and neoplastic and nonneoplastic conditions.
- •
Hemodynamic parameters obtained from perfusion imaging can guide clinical decision making for treatment-related processes, such as radiation necrosis.
Introduction
Neuroimaging plays an essential role in the initial diagnosis and continued surveillance of intracranial neoplasms, with multiple studies showing the utility of perfusion imaging in assessing tumor physiology and hemodynamics. Although conventional MR imaging techniques are useful in evaluating the anatomy and structure of the brain, advanced imaging approaches can provide useful information about physiology and function not visible on the anatomic images. Specifically, perfusion imaging can estimate cerebral blood flow (CBF) and cerebral blood volume (CBV) as markers of angiogenesis within intracranial tumors, can generate transfer constant (k-trans) as a permeability marker in tumors before and after treatment, and can help to distinguish between tumor and treatment effect in previously treated tumors. To date, the most useful perfusion parameters in the clinical neuro-oncology setting are CBF and CBV, which are acquired with exogenous contrast agents (eg, dynamic susceptibility contrast [DSC] MR imaging and iodinated contrast-enhanced computed tomography [CT] perfusion imaging) or without contrast agents (eg, arterial spin labeling [ASL] imaging). In this article, we review the clinical relevance and implications of perfusion imaging in neuro-oncology and highlight promising perfusion biomarkers.
Computed tomography perfusion
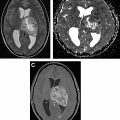
CT perfusion provides information on brain hemodynamics by analyzing the first passage of an intravenous contrast bolus through the cerebral vessels. Raw images are acquired on a multislice CT scanner and are subsequently post-processed by software to generate hemodynamic perfusion maps. This permits quantitative and qualitative assessment of CBV and CBF ( Fig. 1 ). CBV refers to the volume of blood within a given region of brain tissue and is measured in milliliters per 100 g of brain tissue. A closely associated and often interchangeably used term is relative cerebral blood volume (rCBV). This accounts for capillary permeability by measuring CBV relative to an internal control (such as the contralateral normal-appearing white matter) and is expressed as an overall ratio. CBF refers to the amount of blood per unit time passing through a given region of brain tissue and is measured in milliliters per 100 g per minute of brain tissue. Fractional tumor burden (FTB) is a newer perfusion-derived metric and is defined as the volume fraction of tumor voxels higher or lower than a specified CBV threshold.
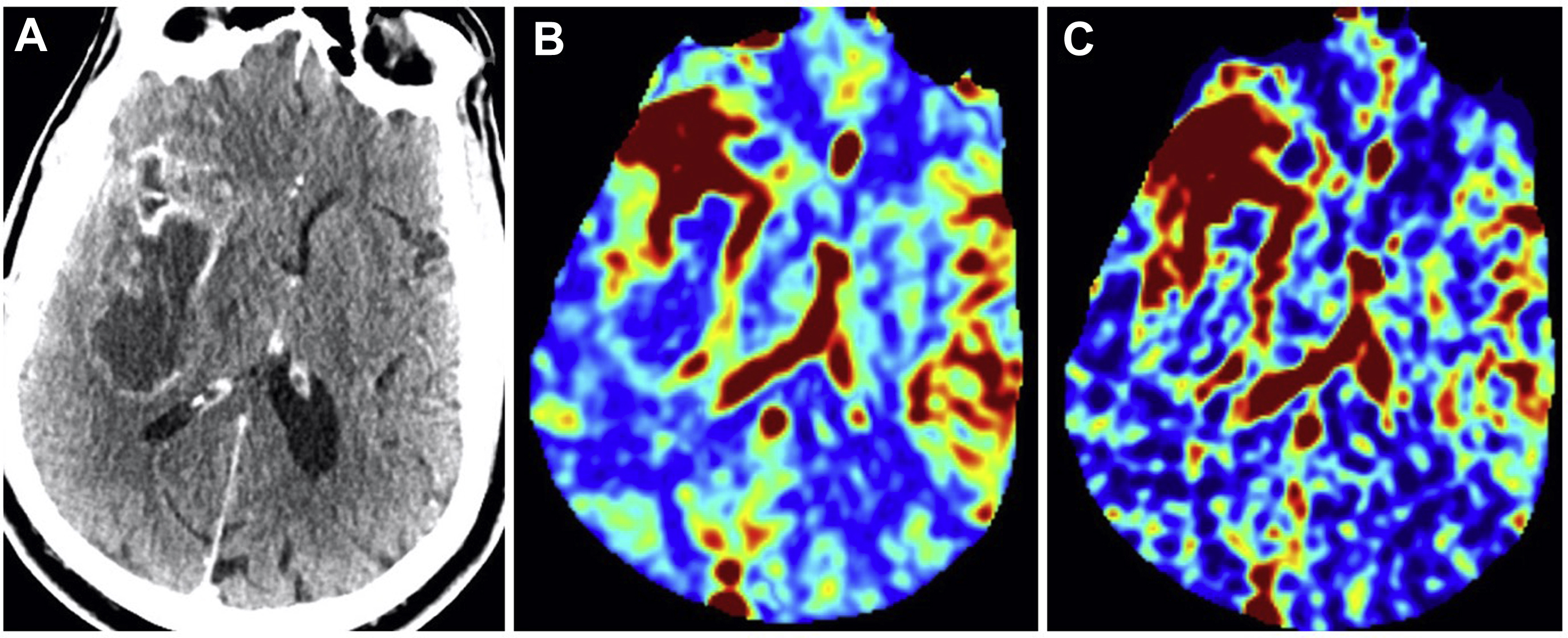
An advantage of using CT perfusion over perfusion MR imaging is the linear relationship between iodine concentration and attenuation on CT, providing a more absolute measurement of vascular parameters. CT also has the benefit of wider availability, faster scanning times, and lower cost compared with MR imaging. CT can also be used in patients with contraindications to MR imaging, such as in those with medical implants. However, CT does require radiation exposure to the patient, which may be additive if serial imaging is needed. In addition, soft tissue resolution on CT is inferior to MR imaging.
Perfusion MR imaging
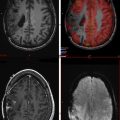
Perfusion MR imaging techniques take advantage of endogenous or exogenous tracers. With regards to exogenous agents, perfusion MR imaging is based on the concept of following an injected bolus of contrast agent over time, which is then used to investigate the perfusion characteristics of brain tumors. Contrast-enhanced perfusion imaging is accomplished with DSC and dynamic contrast-enhanced (DCE) imaging. With regards to endogenous agents, ASL imaging can be used; this technique magnetically labels spins using protons within arterial blood to estimate CBF. In the next paragraphs, we briefly review the techniques of DSC, DCE, and ASL and highlight the clinically relevant parameters acquired from each technique for brain tumor imaging ( Fig. 2 ).
Dynamic Susceptibility Contrast
DSC MR imaging uses a bolus tracking technique that is most frequently based on T2 (spin echo) or T2∗ (gradient echo imaging) effects before, during, and after administration of a gadolinium-based contrast agent. In DSC imaging, injection of a contrast agent causes a transient drop in signal intensity reflective of the effects of the paramagnetic contrast agent. The spin echo technique has the advantage of minimizing brain, bone, and air interfaces and is more sensitive to signal changes from contrast material passage through small vessels. The main disadvantage of spin echo imaging is the requirement of a higher dose of contrast, which is needed to produce signal changes comparable with gradient echo imaging. Gradient echo DSC is faster in terms of image acquisition and takes advantage of first-pass imaging and magnification of contrast-induced signal loss through susceptibility-weighted images. A disadvantage of this technique, however, is related to inherent susceptibility artifacts produced by blood, calcification, or larger vessels. Nonetheless, with either approach, contrast preloading, gamma variate curve fitting, or other leakage correction methods are commonly used to reduce T1 relaxation effects and to account for residual T2/T2∗ effects. , Much like CT perfusion, DSC allows for the calculation of multiple perfusion parameters, such as CBF, CBV, and FTB.
Dynamic Contrast Enhancement
DCE MR imaging uses bolus tracking on T1-weighted imaging, where permeability characteristics of brain tumors are assessed. Advantages of DCE include acquisition with a lower contrast dose and better temporal resolution compared with that of T2- or T2∗-weighted DSC imaging. This is because T1-weighted imaging-based DCE measures the relaxivity effects rather than the susceptibility effects of the injected dose of a paramagnetic contrast agent. The relaxivity effect refers to the generated signal of T1 shortening related to relaxation time, which is inherently stronger than susceptibility. Also, shorter injection times may result in better quantitation of CBV and CBF provided that the temporal resolution of the pulse sequence can allow for dynamic tracking of the injected contrast bolus over multiple time points to extract and estimate T1 signal and concentration. However, DCE suffers from the necessity of advanced and complex pharmacokinetic modeling to account for the nonlinear relationship between the acquired signal intensity and contrast concentration. Nonpharmacokinetic model-based analyses with DCE have attempted to avoid this problem but have unclear physiologic and clinical basis and utility.
Despite several choices for modeling, the Extended Tofts Model, which is a two-compartment model of the vascular and extracellular-extravascular spaces, is frequently used in brain tumor imaging. In this model, the leakage of contrast is measured quantitatively and a triexponential enhancement curve is fitted to a theoretic model based on compartmental analysis. This allows for many parameters to be obtained, such as the k-trans, volume of the extravascular extracellular space, and blood plasma volume. Of these, k-trans has been the most widely used and investigated in clinical neuro-oncology. It is important to know that although k-trans is thought to be a marker of permeability, it is more reflective of blood flow in certain conditions. For example, in situations where there is high permeability, the flux of gadolinium-based contrast agent is primarily limited by flow and, therefore, k-trans primarily reflects blood flow. In situations where there is low permeability, the contrast leakage is limited in its ability to flow into the extravascular-extracellular space and, therefore, k-trans primarily reflects permeability.
Arterial Spin Labeling
ASL imaging is a noncontrast technique that takes advantage of an endogenous tracer by magnetically labeling spins using protons within arterial blood. , The MR imaging sequence acquisition is built in such way that a delay allows the labeled water molecules to flow into the brain tissue and exchange with the brain tissue water, which results in small changes in the magnetization of the tissue water. When evaluated in conjunction with control (unlabeled) images, CBF images are generated after subtraction from the labeled images. Although not yet widely used or established in clinical practice, this technique for acquiring cerebral perfusion offers several advantages over DSC and DCE, primarily because it does not require injection of a gadolinium-based contrast agent. Therefore, ASL is a promising technique for assessing perfusion in patients who have renal dysfunction or severe allergic reactions to gadolinium or who require frequent follow-up contrast-enhanced examinations. However, widespread clinical applications of this method have been limited, in part because of longer acquisition times and sensitivity of the technique to patient motion.
Clinical use of perfusion imaging in neuro-oncology
Although conventional MR imaging is essential for the diagnosis and evaluation of brain tumors, it does not confer much information about tumor vascularity and physiology. Perfusion imaging is valuable because it is used to help grade tumors; differentiate between tumor types; differentiate tumors from nonneoplastic lesions; guide intraoperative sampling; and, most importantly, determine efficacy of treatment. The initial differentiation between neoplastic and nonneoplastic lesions is difficult with conventional MR imaging, often requiring direct tissue sampling for diagnostic confirmation. Perfusion imaging can help to distinguish between infectious and neoplastic lesions, as CBV of infection tends to be significantly lower than CBV of metastases or glioblastomas, likely reflective of their respective vascular densities. However, there is potential for overlap between low-grade tumors and nonneoplastic lesions. Thus, systematic incorporation of perfusion imaging as part of a multiparametric approach, potentially with MR spectroscopy, can aid in improving diagnostic confidence. ,
Grading of Tumors
MR and CT perfusion imaging have shown to be helpful in determining the initial grade of gliomas based on increased or decreased tumor perfusion, with studies showing that specific perfusion metrics correlate strongly with overall histopathologic grade. These perfusion metrics can predict tumor behavior, because tumor aggressiveness and growth are associated with endothelial hyperplasia and endothelial neovascularization. As such, it is not surprising that higher CBV correlates with lesion vascularity and higher tumor grade. However, there is a substantial overlap of perfusion markers in tumors of varying grades and histology, which somewhat limits the discriminatory ability of perfusion imaging in certain tumor types and in differentiating between higher grades of tumors (eg, grade III and IV gliomas). K-trans has shown promise in differentiating between low-grade (grade I) and higher-grade (grade II, III, or IV) tumors, although larger multicenter studies are still needed to validate its use in the clinic.
Differentiation Between Tumor Types
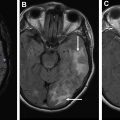
An important and often difficult diagnostic dilemma exists when differentiating between glioblastoma, solitary brain metastasis, and primary central nervous system lymphoma. This is because of their similar and often times overlapping appearance on conventional MR imaging, presenting as solitary and avidly enhancing lesions with peripheral T2 hyperintensity. It is important to distinguish between these entities, because management (surgery and/or chemotherapy) is different. , A study by Neska-Matuszewska and colleagues found that maximum CBV within the tumor core enabled discrimination of less perfused primary central nervous system lymphomas from that of the more hyperperfused glioblastomas and metastases. When discriminating between glioblastoma and metastasis, maximum CBV within the perilesional zone was found to be most helpful. Increased CBV was observed within the peritumoral zones, reflecting the infiltrative and highly vascular nature of glioblastomas. Alternatively, decreased CBV was observed within the perilesional zone of metastases, reflecting regional vasogenic edema rather than nonenhancing infiltrative tumor.
Differentiation Between Tumor and Nonneoplastic Conditions
On conventional imaging, aggressive neoplasms, such as a necrotic glioblastoma, may mimic and may be difficult to differentiate from other entities, such as a cerebral abscess, because these entities can appear as rim-enhancing lesions with regional edema. Perfusion imaging is helpful in these cases, because higher-grade neoplasms tend to have increased neovascularity and capillary density and, therefore, higher CBV, whereas abscesses tend to have significantly lower CBV. Other studies have found that neoplastic lesions also demonstrate higher CBV when compared with infectious lesions. For example, Floriano and colleagues found that a rCBV value less than 1.3 yielded a 92.6% specificity for identifying infectious lesions, adding support for the use of perfusion imaging in distinguishing between infectious and neoplastic brain lesions.
Tumefactive demyelinating lesions can also mimic higher-grade neoplasms, given their aggressive appearance on structural MR imaging. However, it has been demonstrated that tumefactive demyelinating lesions have lower CBV than high-grade gliomas because of the absence of neoangiogenesis, a prominent feature of high-grade tumors. ,
Tumor Sampling
Although the optimal management of low-grade gliomas is surgical resection, watchful waiting is reasonable in certain patients. With watchful waiting, imaging is used to ensure tumor stability over time. If tumor progresses on imaging or shows changes indicative of transformation to a higher-grade lesion (eg, new or increasing enhancement or perfusion), then intervention may be necessary. However, some higher-grade tumors may lack enhancement altogether. In addition, the presence of contrast enhancement may not always indicate a higher-grade tumor because its presence is only reflective of a disrupted blood-brain barrier. Furthermore, although nonenhancing tumors are more likely to be of higher grade in older patients, diagnosis of lower-grade tumors cannot be reliably made without a proper biopsy.
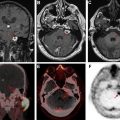
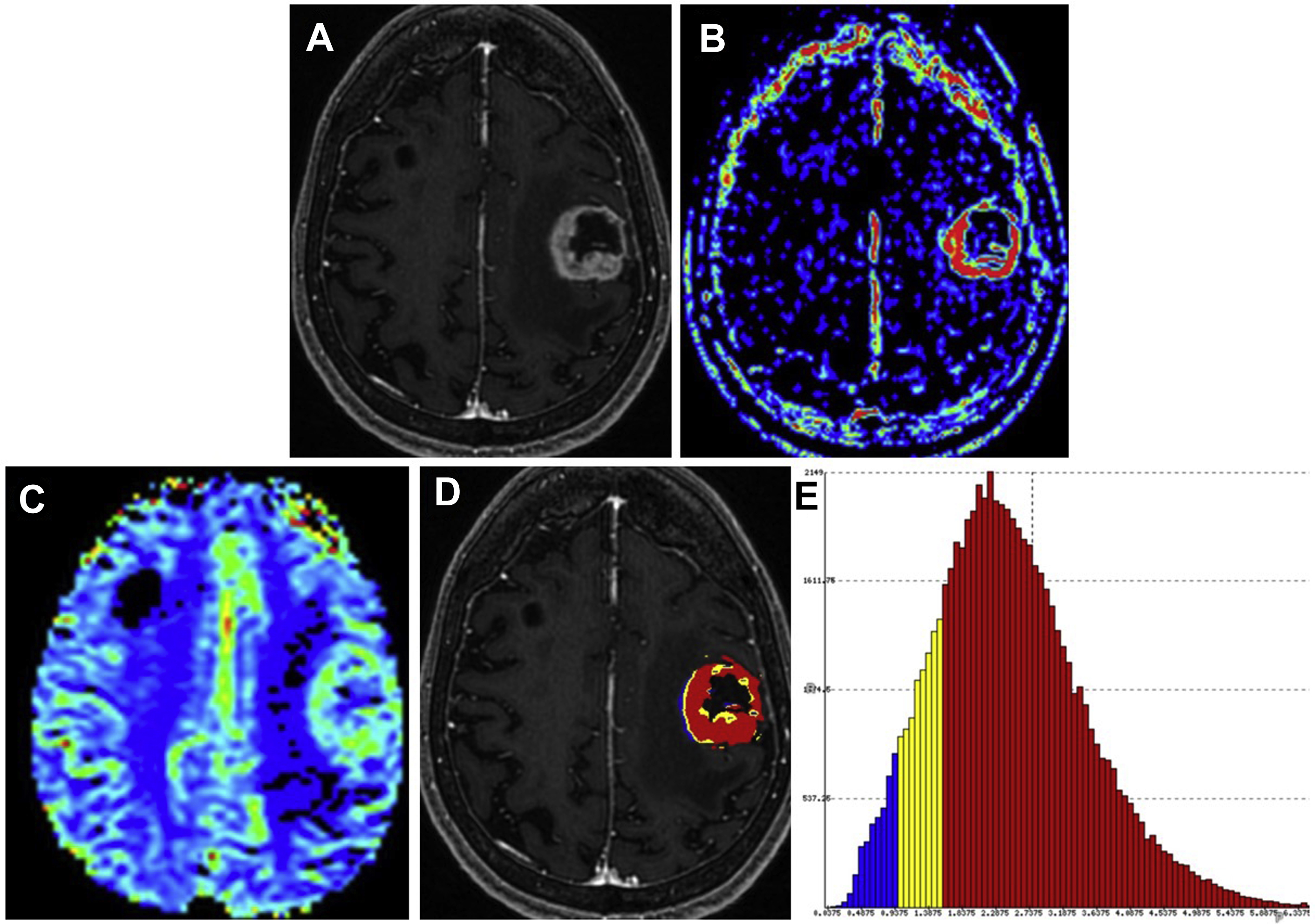
Stereotactic guided biopsy is commonly used for sampling of tumor tissue ( Fig. 3 ). However, because of the internal heterogeneity of brain tumors, sampling error remains a problem. Studies have shown that regions of increased CBV on perfusion imaging can help to guide biopsies in patients with gliomas. CBF and CBV maps are used to identify areas of maximum hyperperfusion within a lesion to guide intraoperative sampling, because these areas are most likely to yield diagnostic tissue representative of the highest grade component of tumor. , Beyond CBV, the use of FTB has shown the highest correlation with actual tumor content, further supporting that perfusion MR imaging can potentially reduce sampling error in histopathologic diagnosis and improve target selection for stereotactic biopsy. ,