CHAPTER 14 Clinical Techniques of Cardiac Magnetic Resonance Imaging
Morphology, Perfusion, and Viability
TECHNICAL REQUIREMENTS: MOTION COMPENSATION
The ability to compensate for motion, both cardiac and respiratory in origin, is critical to successful MR imaging of the heart. As newer generations of cardiac scanners, with software and hardware advances, have become increasingly available, the motion obstacle can be better resolved. One of the most important obstacles for MRI to overcome has been the ability to image quickly enough to overcome the limited window of opportunity to produce snapshot images of a motionless heart. The implementation of electrocardiographic gating remains one of the most significant advances to facilitating imaging in this respect. This has been further optimized by the addition of k-space segmentation (Fig. 14-1).
In electrocardiographic triggering, data acquisition is synchronized to the cardiac cycle. The R wave is used as a starting point from which a defined trigger delay can be timed to acquire a portion of k-space. The trigger delay is typically set at mid-diastole, where minimal motion occurs.1 This partitioning allows each package of data to be acquired in a short time interval, ideally less than 50 ms, and therefore without motion degradation. In k-space segmentation, the full complement of k-space data is compiled by imaging over several successive cardiac cycles, each of which contributes its respective portion of information. Electrocardiographic triggering can be implemented in a prospective fashion, where the R wave is an actual trigger for data acquisition. More often, however, retrospective triggering is used, in which the R wave is a marker for sorting information already acquired through the entire R-R interval. The latter allows complete diastolic phase imaging, which is important in accurate functional assessment for which end-systolic and end-diastolic views of the cine sequence are required.
Electrocardiographic gating may be impaired in patients with large thoracic cavities, such as in emphysema, or when large pericardial effusions decrease the electrical signal. Another artifact, the magnetohydrodynamic effect, occurs when ions contained within blood pass through the magnetic field, inducing a voltage that distorts the electrocardiographic tracing. This particular limitation can be minimized with an optimized version of gating available through vector electrocardiography, which is less prone to distortion of the cardiac tracing by the magnetohydrodynamic effect.2
The second significant motion hurdle to overcome has been the elimination of respiratory artifacts. This has been addressed in abdominal imaging by using breath-holding during image acquisition, when possible, and has been adapted to MRI as well. Although this produces excellent image quality with fast imaging techniques such as turbo field echo and echo-planar imaging, there remain limitations. Breath-hold reproducibility can be low, resulting in potential slice misregistration in multislice acquisitions. This is particularly problematic in left ventricular function assessment on short-axis cine imaging. Instructing patients to breath-hold at end-expiration has been shown to provide the most consistent data and to minimize variability in position.3 Breath-hold imaging is also limited by the amount of time that can be spent on other aspects of the image quality, such as the signal-to-noise ratio (SNR) and spatial and temporal resolution. This limitation is in part offset by parallel imaging techniques that can speed up imaging and provide magnetic resonance (MR) currency of time, which can be used for optimization of other imaging parameters.
More recently, an alternate method for respiratory compensation has been developed in the form of the navigator echo technique (Fig. 14-2). First described by Ehman and Felmlee,4 this technique uses an interleaved column of excitation perpendicular to the direction of motion for assessment of tissue displacement. The navigator echo is typically prescribed at interfaces with high tissue contrast; such as the right hemidiaphragm, where signal is compared to a reference echo for displacement. Information on displacement can then be used to select echoes for image reconstruction that reflect nonmotion. The scan efficiency depends on operator-dependent factors such as stringency of gating criteria, with windows of 3 to 5 mm being typical for acceptance or rejection of echo information. The larger the acceptance window, the higher the efficiency and shorter overall acquisition time, but with consequent increase in motion blurring. Other factors that can be manipulated in the navigator technique include prospective versus retrospective acquisition and the number of navigator echoes acquired, with a resulting balance between image quality and time efficiency. By avoiding breath-holding in this technique, patient comfort and compliance are improved. One of the primary advantages of the navigator technique is the ability to spend time currency to improve SNR and spatial resolution.
Limitations to navigator techniques include respiratory drift of the diaphragm beyond the acceptance window, which occurs in patients who fall asleep or are anxious during the early part of an examination but later settle into a different rhythm.5 Careful monitoring of patients’ breathing patterns and keeping them alert during the scan acquisition can minimize respiratory drift. Further improvements have been made to the navigator technique specifically for coronary MR angiography (MRA) by phase ordering.
TECHNIQUES
Anatomic Overview
Indications
Cardiac examinations generally begin with a morphologic overview of the heart, pericardium, mediastinum, and great vessels. T1-weighted images provide an excellent overview of this anatomy and thus remain standard in most MRI protocols. Tissue characterization can also be performed on T1-weighted images, looking for fat in entities such as arrhythmogenic right ventricular dysplasia or cardiac mass lesions. T2-weighted images, which can further characterize focal masses, are now demonstrating increased usefulness for several clinical diseases, often reflecting edema in the myocardium. This can be especially helpful in ischemic heart disease, in which differentiation of acute from chronic infarcts, which can look similar on viability sequences (see later), can be made by the presence of a bright T2 signal in the acute scar.6 Similarly, T2 edema can be identified in areas of disease involvement in acute myocarditis7 and nonischemic cardiomyopathy.8
MRI has also been demonstrated to be useful in the noninvasive assessment of cardiac iron overload. Patients with thalassemia, for example, may have transfusion-related iron overload. The presence of iron has prognostic implications for these patients with an increased risk of heart failure. Iron deposition in the heart is also an important cause for mortality in this patient population compared with other organs. Early detection can be difficult by serum ferritin measurements, which are not necessarily reflective of myocardial content, and routine biopsy of the myocardium is not feasible. T2*-weighted imaging can provide noninvasive measures of iron deposition in the myocardium. with correlation to deterioration in ventricular function.9
Technique Description
Turbo spin-echo (TSE) sequences are most commonly used for routine morphologic assessment. In contrast to other anatomic locations, the TSE sequence in cardiac imaging is modified by the implementation of a dual inversion preparatory pulse that produces black blood (BB) images. The absence of signal in the blood pool is achieved by using a selective and nonselective 180-degree inversion pulse, which is followed by a long inversion time chosen to null blood magnetization (Fig. 14-3). These preparatory pulses are followed by a gated TSE readout with k-space segmentation. This can be performed with either breath-holding or free-breathing.
The black blood pulse produces good myocardium–blood pool differentiation. These static images are of high SNR, with good tissue contrast (Fig. 14-4). Furthermore, image parameters are easily manipulated to allow for T1- or T2-weighted tissue characterization. If fat saturation is required, an additional selective 180-degree inversion pulse can be added with a short inversion time to null fat, also known as STIR (short tau inversion recovery).
Pitfalls and Solutions
Slow-flowing blood can result in poor blood suppression, producing an endocardial border of hyperintensity on T2-weighted images. When a thin layer of pseudo-T2 involvement is isolated to the endocardium, correlation should be made to other imaging sequences to help determine the true anatomic location of signal changes. If the changes are determined to be adjacent to the endocardium, then they are likely artefactual. This artefact is particularly notable in regions adjacent to wall motion abnormality, at the ventricular apex, or the interstices of trabeculae.11
Perfusion
Indications
Myocardial perfusion is important in determining the hemodynamic significance of coronary artery stenoses identified at angiography. Nuclear cardiology currently fulfills the role of perfusion assessment by single photon emission computed tomography (SPECT)12 and positron emission tomography (PET) imaging.13 However, both techniques share the relative limitation of spatial resolution and radiation exposure. SPECT imaging also suffers from soft tissue attenuation, whereas access to PET imaging, despite its usefulness, is limited at this time.
Cardiac MR strengths include good tissue contrast, spatial resolution, and temporal resolution, which make it a natural candidate for perfusion imaging. First-pass perfusion MRI with gadolinium-based contrast agents is an accepted tool in the evaluation of coronary artery disease (Fig. 14-5).14 When perfusion MRI is compared with current scintigraphic techniques, it performs well.15 Overall, numerous studies evaluating the performance of MR perfusion in detecting obstructive coronary artery disease produce sensitivity and specificity of 83% and 82%, respectively.16
Contraindications
Perfusion imaging is not performed if the patient cannot receive the stress agent. Table 14-1 lists contraindications to pharmacologic stress agents. General contraindications to MR examination and to gadolinium-based contrast agents, including nephrogenic systemic fibrosis are also reasons that preclude cardiac MR perfusion examinations.
TABLE 14-1 Contraindications to Pharmacologic Stress Agents
| Agents | Contraindications |
|---|---|
| Dobutamine | |
| Adenosine, dipyridamole |
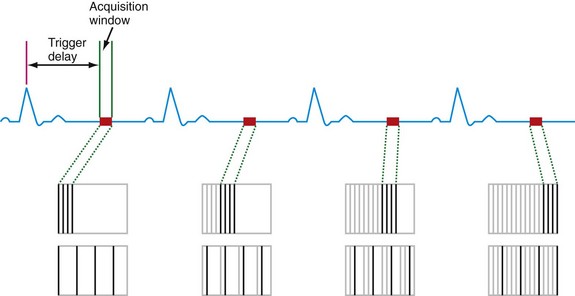
 FIGURE 14-1
FIGURE 14-1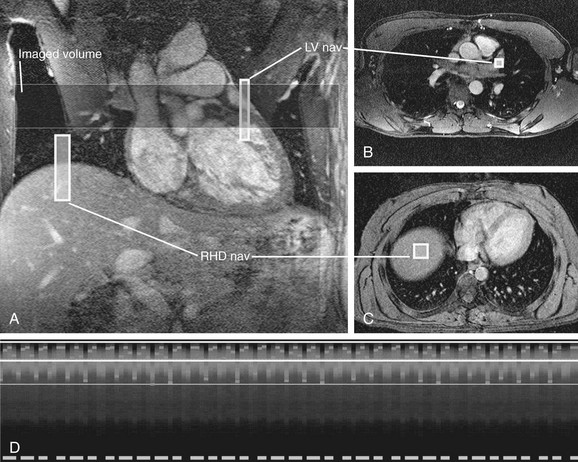
 FIGURE 14-2
FIGURE 14-2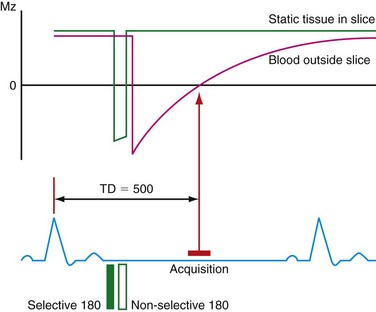
 FIGURE 14-3
FIGURE 14-3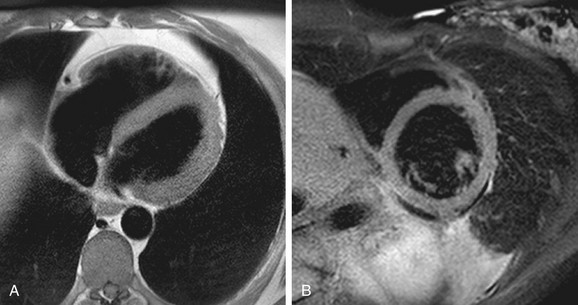
 FIGURE 14-4
FIGURE 14-4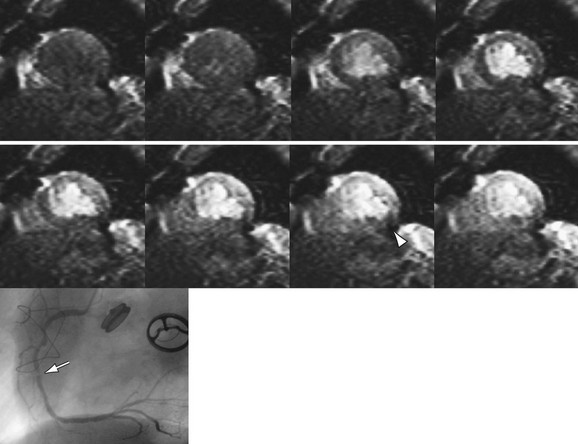
 FIGURE 14-5
FIGURE 14-5


