CHAPTER 38 Coarctation of the Aorta
Definition
Coarctation refers to a stenosis of the proximal descending thoracic aorta that is almost always opposite the insertion of the ductus arteriosus—that is, at the junction of the distal aortic arch and the descending aorta just below the origin of the left subclavian artery (Fig. 38-1).1–4
Prevalence and Epidemiology
Coarctation of the aorta occurs in 3.2 of 10,000 births and accounts for 5% to 10% of all cases of congenital heart disease.1–4 Coarctation is more common in white males than in white females, with a male-to-female ratio of 1.3 to 2.0 : 1.3 Most cases are sporadic, but there may be a genetic inheritance.
Etiology and Pathophysiology
Coarctation is thought to be the result of a malformation of the aortic media, leading to a posterior infolding or shelf.1,5,6 Most often, the shelf is discrete and opposite the ductus arteriosus. However, the malformation may be a long segment and circumferentially surround the aorta. In the latter form, there is typically diffuse tubular hypoplasia of the transverse arch and isthmus.1,7,8
Histologic examination shows thickened intima and media that protrude posteriorly and laterally into the aortic lumen. The ductus arteriosus or ligamentum arteriosus inserts anteromedially at the same level.1
In milder forms of coarctation, the left ventricular myocardium hypertrophies to normalize myocardial wall stress and maintain normal systolic ventricular function. Left ventricular ejection fraction is often normal to increased. Collateral vessels also develop, which act as a source of lower body perfusion and help maintain normal flow to abdominal viscera.1
MANIFESTATIONS
Clinical Presentation
Critical or severe coarctation manifests in the first few days to first week of life. Symptoms include cyanosis (especially of the lower body), heart failure, shock, multiorgan failure and necrotizing enterocolitis.1–5
Milder forms of coarctation manifest later in life. Because left ventricular function is maintained and cardiac outflow is good, patients come to clinical attention because of hypertension or a heart murmur related to a bicuspid aortic valve.1–4 Blood pressure in the upper extremities is typically higher than that in the lower extremities. However, if there is an aberrant right subclavian artery, the pressure in the right upper arm may be equal to or lower than that in the left upper arm.
Imaging Studies
Techniques and Findings
Ultrasound
Two-dimensional echocardiography may show the site and extent of coarctation in young infants. Doppler imaging can help in determining the hemodynamic severity of the obstruction. In general, images in infants are of higher quality than those in older children and adults. In the latter populations, images of the distal arch may be suboptimal, requiring additional imaging with CT or MRI.1,2,4
Computed Tomography
CT angiography is performed with a pulmonary embolism protocol using thin collimation (<1 mm), pitch lower than 1.5,9,10 fast scan time, and a single breath-hold, when possible. In adolescents and adults, contrast medium is injected via a power injector at a high flow rate of 3 to 4 mL/sec using a contrast volume of 100 to 150 mL (280 to 320 mg I/mL). Scan delay time can be determined with an automatic bolus tracking system with the cursor over the aortic isthmus or empiric timing. Empiric timing, using a delay of 20 to 25 seconds after the start of the injection, is a default method if the bolus tracking fails to trigger. Electrocardiographic (ECG) gating is not needed for the evaluation of coarctation.
Special Considerations for Pediatric Patients
The contrast volume is 2 mL/kg (not to exceed 125 mL). Intravenous contrast medium can be administered with a power injector or manual (hand) injection. A power injector is used when a 22-gauge or larger cannula can be placed in an antecubital vein. For a 22-gauge catheter, flow rates are 1.5 to 2.5 mL/sec.10,11 Flow rates for larger gauge catheters are similar to those described above. With a manual injection, the contrast is pushed as quickly as possible. Determination of the scan initiation time can be made by an empiric or bolus tracking method. In pediatric patients weighing less than 10 kg, an empiric scan delay of 12 to 15 seconds after the start of the intravenous contrast injection suffices.10 In larger patients, the delay time is 15 to 25 seconds.
Magnetic Resonance Imaging
MRI, like CT, can accurately define the location, extent, and degree of aortic narrowing, collateral vessel formation, and valvular and ventricular morphology. In addition, MRI can provide an assessment of coarctation physiology, including information on blood flow and the pressure gradient across the stenosis.1,3 Because MRI yields functional information and does not rely on ionizing radiation, many centers prefer this modality over CT, especially for children.
Noncontrast-enhanced methods, both black blood and bright blood techniques, and three-dimensional contrast-enhanced MR angiography (CEMRA) are routinely acquired. CEMRA is performed by acquiring a three-dimensional gradient-echo sequence following a rapid bolus of gadolinium. There are several ways to time contrast delivery. The best guess method is the simplest, the automated detection method is the most operator-independent,12 and the timing run is the most reliable method.13
Velocity-encoded (VEC) phase contrast techniques are used to determine flow, peak flow rates, and the volume of flow per unit time (see Chapter 17, Fig. 17-8).14,15 The pressure gradient across the obstruction is calculated with a modified Bernoulli equation (product of the cube of the peak velocity as measured in meters per second).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


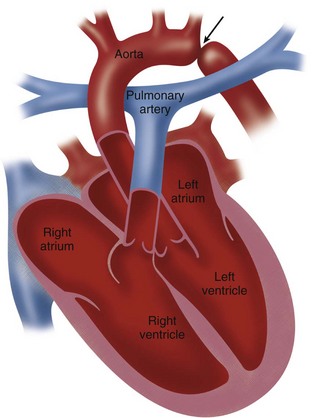
 FIGURE 38-1
FIGURE 38-1