Anatomy and Anatomical Associations
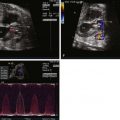
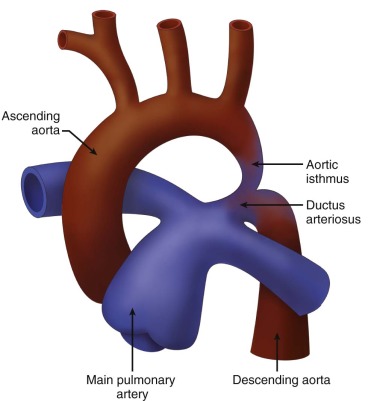
Coarctation of the aorta is a narrowing of the lumen of the aortic arch. There are two general types: (1) juxaductal, or “adult-type” coarctation, is discrete narrowing at the isthmus, the region of the aortic arch distal to the take-off of the left subclavian artery, and (2) “infantile type,” or tubular hypoplasia. In this form of aortic arch obstruction, there is long segment narrowing of the transverse arch and it is often associated with intracardiac defects. Practically, most forms of coarctation seen in the perinatal period are a combination of these two, where there is an element of hypoplasia of the isthmus and then a discrete narrowing as one approaches the insertion into the ductal arch ( Figure 20-1 ). Abdominal coarctation is a rare finding of narrowing of the descending aorta at the region beneath the diaphragm, commonly at the level of the renal arteries.
Anatomical associations are common in the fetus with aortic arch obstruction. One of the most common findings, and one that typically tips the fetal heart examiner off to the possibility of coarctation of the aorta, is left ventricle (LV)–to–right ventricle (RV) size discrepancy or disproportion, in which the LV cavity appears much smaller and the RV cavity much larger than expected. This disproportion is noted in the face of no ostensible mitral or aortic valve disease.
Ventricular septal defects are often present in coarctation and can be of the conal/infundibular posterior malalignment, conoventricular, or muscular type. Coarctation can occur in the setting of more complex cardiac lesions such as hypoplastic left heart syndrome (HLHS), complete atrioventricular canal, and transposition of the great arteries.
Frequency, Genetics, and Development
Coarctation of the aorta occurs in 0.3 per 1000 live births and accounts for 6% to 8% of infants with congenital heart disease. As with other forms of left-sided obstructive lesions, coarctation occurs more frequently in males than in females. There is a known association with Turner’s syndrome (45,XO) in both complete and mosaic forms. Coarctation occurs in 35% of patients who are affected by Turner’s, and a documented familiar recurrence suggests a genetic substrate.
The precise developmental mechanism remains unknown, but two theories exist. The hemodynamic theory suggests that an associated defect such as a bicuspid aortic valve, or some other impetus, drives blood away from the developing LV and reduces the amount of flow in the ascending aorta and across the aortic arch. This decrease in antegrade flow across the aortic arch leads to diminished growth and formation of a narrowed segment. In fact, the isthmus of the aorta normally receives the smallest proportion of cardiac output of any structure arising from the fetal heart. The LV ejects 40% of the entire fetal cardiac output; by the time flow reaches the isthmus after the head and neck vessels have been perfused, only 10% to 15% remains to cross the isthmus and join with the ductus arteriosus and descending aorta. Therefore, even a small reduction in aortic flow can have a substantial impact on the reduction of blood flow crossing the aortic isthmus. The ductal tissue theory suggests that actual ductal tissue migrates abnormally into the aortic arch and then constricts after ductal closure, leading to a coarctation.
Prenatal Physiology
In the normal fetal circulation, the dominant RV supplies output to the body via the ductus arteriosus. The left heart fills primarily via right-to-left shunting at the foramen ovale and the LV supplies output to the head and neck vessels, with only 10% to 15% of the fetal cardiac output traversing the aortic isthmus. Therefore, the fetal aortic isthmus often normally appears relatively small with tapering at the region of ductal insertion. Further abnormal narrowing in the isthmus as seen in coarctation is well tolerated in the fetus because the large ductus arteriosus allows for flow around any narrowed region, providing unobstructed perfusion to the body. There is typically normal growth and development, with the fetus reaching full term.
The region of the isthmus is quite interesting because it is the bridge between the cephalic and the subdiaphragmatic arterial circulations. Fouron has written extensively about the importance of evaluation of the aortic isthmus through Doppler sampling. Analysis of flow patterns within the isthmus can provide a wealth of information concerning the relative resistances of the upper and lower fetal body, which change dramatically during periods of stress and unwellness (see Chapter 3 ).
Prenatal Management
When clear-cut, severe hypoplasia of the isthmus is seen on two-dimensional imaging, the diagnosis of coarctation is straightforward. However, because the aortic isthmus is normally a relatively small structure, in the presence of a normal large patent ductus arteriosus, the diagnosis can be quite challenging. LV-RV size discrepancy may suggest a coarctation; however, when looking at the isthmus, it may appear proportionately small but of good size relative to the LV and ascending aorta. Furthermore, LV-RV size discrepancy can be seen in a number of conditions besides coarctation of the aorta ( Box 20-1 ). A left superior vena cava to the coronary sinus may theoretically impair filling of the LV by altering left atrial compliance and reducing right-to-left shunting at the foramen ovale. Interruption of the inferior vena cava with azygos continuation to the right superior vena cava results in a redirection of inferior vena caval flow because the normal streaming pattern across the foramen ovale caused by the inferior vena cava entering the floor of the right atrium is averted. The RV is dilated and can appear volume loaded in this condition because both inferior and superior venous return drains via the superior vena cava and is directed caudad across the tricuspid valve. Anomalies of pulmonary venous return, with connection and drainage away from the left atrium, may reduce LV filling and create the impression of a relatively underfilled LV. We have also seen situations in which LV-RV disproportion predicted the presence of a genetic syndrome or a neurological condition, without any venous anomaly or postnatal evidence for arch narrowing. Finally, there are cases of LV-RV size disproportion in which postnatal findings from a somatic, genetic, and cardiovascular perspective are completely normal.
- •
Coarctation of the aorta
- •
Bicuspid aortic valve
- •
Structural mitral valve disease resulting in limitation of flow across (e.g., mitral stenosis, supravalvar mitral ring)
- •
Left superior vena cava to the coronary sinus
- •
Interruption of the inferior vena cava with azygos continuation to the right superior vena cava
- •
Total anomalous pulmonary venous connection
- •
Partial anomalous pulmonary venous connection
- •
Genetic syndromes (e.g., Turner’s syndrome)
- •
Central nervous system anomalies
- •
Normal fetus
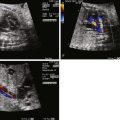
Prenatal diagnosis of coarctation of the aorta is important because it has implications for immediate postnatal management and site of delivery. In order to better discriminate between LV-RV disproportion due to coarctation of the aorta versus other causes, a number of investigators have attempted to develop predictive criteria. First, it must be established whether one is dealing with the other end of the spectrum, in which there is a nonviable inadequate LV and HLHS. In HLHS, the LV cavity is quite small and dysmorphic in shape and appearance. One of the key diagnostic hallmarks is an associated continuous left-to-right shunt at the atrial level. This is not the condition we are speaking of when referring to the LV-RV size disproportion phenomenon in this chapter. In LV-RV size disproportion, the LV cavity appears small in its width and looks underfilled; however, it typically reaches greater than two thirds the distance toward the apex of the heart. Shunting at the atrial level is typically the normal right to left; however, it may be bidirectional. Parameters related to relative size of structures within the heart and great vessels as well as unique Doppler flow patterns have been described, which can help discriminate those that will require intervention for coarctation of the aorta after birth ( Box 20-2 ).
- •
Visualization of a shelf, or discrete narrowing of the aorta in the region of the isthmus as it inserts into the ductal arch
- •
Visualization of continuous, persistent forward flow in the isthmus on color Doppler imaging
- •
Low-velocity persistent forward flow with low pulsatility index on pulse wave Doppler imaging
- •
Aortic isthmus Z score less than –2 on “three vessels and trachea” view
- •
Persistent absence of “catch-up” growth of the aortic isthmus, where serial measures continue to reveal aortic isthmus at Z score less than –2
- •
Isthmus–to–ductus arteriosus diameter ratio less than 0.74 on “three vessels and trachea” view
- •
Transverse aortic arch diameter measurement of less than 3 mm at greater than 30 weeks’ gestation
- •
Aortic annulus–to–pulmonary annulus diameter measurement less than 0.6 in the outflow tract views
- •
Mitral valve–to–tricuspid valve annulus measurement in four-chamber view less than 0.6
- •
Main pulmonary artery–to–ascending aorta diameter ratio of greater than 1.6, when measured in the mediastinum
- •
Bidirectional shunting at the level of the atrial septum
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree