COCHLEOVESTIBULAR NERVE AND CEREBELLOPONTINE ANGLE TUMORS AND CYSTS
KEY POINTS
- Magnetic resonance imaging and occasionally computed tomography are performed routinely to look for abnormalities of the cochleovestibular nerve or cerebellopontine angle.
- Pathology in this region may be diverse, but the vast majority of cases are straightforward diagnostic situations and imaging findings typically drive medical decision making.
Cochleovestibular nerve (CVN)-origin neurogenic tumors and meningiomas account for the vast majority of CVN and cerebellopontine angle (CPA) masses. Epidermoid cysts and arachnoid cysts make up the majority of other masses.
Beyond those tumors and cysts, other masses are very uncommon and include metastatic disease to the meninges or CPA; perineural spread of lymphoma or other cancers; and less usual extra-axial tumors such as paraganglioma, chordoma and chondrosarcoma, hemangiopericytoma, and primary meningeal melanoma. Inflammatory and vascular lesions of the CVN and CPA are discussed in Chapters 133 and 135, respectively.
ANATOMIC AND DEVELOPMENTAL CONSIDERATIONS
Embryology
Theoretically, CVN schwannomas arise from the developmental zone of transition of central myelin to peripheral myelin. This zone is generally located in the outer third of the internal auditory canal (IAC); thus, early lesions of the CVN will often be first visible in that location. They may arise elsewhere, so that particular theory of site of origin should not be overemphasized. These lesions usually arise from the vestibular nerve and are most properly called vestibular schwannomas.
The origins of epidermoid cysts from epithelial rests are discussed in Chapter 8. Arachnoid cysts occur during the development of the arachnoid membrane, possibly due to the formation of diverticuli that may also incorporate choroid, secretory neuroepithelium, or arachnoid granulations that can produce cerebrospinal fluid (CSF) or secretions and contribute to cyst expansion.
Applied Anatomy
A diligent search of the entire course of the CVN is essential for evaluating imaging studies performed in patients with symptoms referable to the nerve. Exclusion of causative pathology with a high degree of confidence is the usual goal of the study.
In order to accomplish such a search, the computed tomography (CT) and magnetic resonance imaging (MRI) anatomy of the CVN must be completely understood, including its brain stem nucleus, its course through the brain stem, its cisternal segment, its course through the IAC, and points of distribution of its three main branches to the cochlea and vestibular system. This anatomy is presented in detail in Figures 104.11 through 104.14 and Chapter 104.
IMAGING APPROACH
Techniques and Relevant Aspects
In general, MRI and CT in this anatomic region almost always require the highest possible spatial resolution. These factors used to develop optimal protocols are discussed in detail in Chapters 1 through 3. Specific protocols for magnetic resonance (MR) and CT studies for investigating CVN and CPA problems in general appear in Appendixes A and B. Any such MR study must include a three-dimensional steady state acquisition with nominal section thickness 1 mm that can be analyzed in any plane. The same type of CT data analysis is necessary.
The CVN and CPA are typically studied with dedicated contrast-enhanced studies of the temporal bone. These studies must also include definitive high-resolution images of the posterior fossa and CVN. MR studies should also always include images that are as definitive as possible for evaluating the membranous labyrinth of the inner ear and related structures such as the vestibular aqueduct. The inner ear is the “end organ” of the CVN innervation, and its status will almost always be in question when potential pathology of the CVN and CPA is suspected. Non–contrast-enhanced CT only may be used to evaluate the inner ear anatomically, but that is the subject of pathologic processes that are discussed in other chapters even though they may be functionally related to CVN and CPA problems.
Pros and Cons
MRI should be done first, as it most confidently excludes causative pathology at all CVN segments (i.e., the brain stem, cisternal, IAC, and inner ear segments) from its brain stem nucleus to the inner ear.1–5 MRI is more sensitive than CT for excluding intra-axial pathology such as demyelinating disease, pia-arachnoid diseases, and small neoplasms of the nerve that might involve the cisternal segment or the segment within the IAC (Fig. 134.1) as well as perineural spread of cancer.
CT may be done as a supplement to most confidently exclude other pathology that might mimic that of the CVN such as superior semicircular canal dehiscence. This supplemental imaging may be done as a routine study or when MRI findings are suspicious for an anatomic abnormality or are related to bone, such as otosclerosis that creates a mixed hearing loss.
Controversies
Controversies with regard to the evaluation of the CVN are presented in Chapter 104.


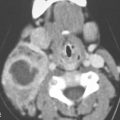
FIGURE 134.1. Computed tomography (CT) versus magnetic resonance imaging (MRI) for the depiction of intracanalicular and cerebellopontine angle (CPA) disease. A: On contrast-enhanced CT, an inhomogeneous enhancing mass is seen in the left CPA with suspected extension into the internal auditory canal (IAC) (arrow). B, C: On three-dimensional steady state (B) and contrast-enhanced T1-weighted (C) images, the partially cystic vestibular schwannoma is more easily detected and the precise extent into the CPA and IAC can be better delineated. D, E: Erosion of the posterior wall of the IAC (arrow in D) caused by a large, partially cystic and mainly extracanalicular schwannoma as seen on MRI (E). Note how the mass has “trapped” the trigeminal cistern (arrow in E), causing it to expand. (continued)
SPECIFIC DISEASE/CONDITION
Tumors of the Cochleovestibular Nerve and Cerebellopontine Angle
Etiology
Neurogenic tumors and meningiomas associated with neurofibromatosis (NF) type 1, NF type 2, or mosaics of the phacomatoses make up a small percentage of the tumors of the CVN and CPA. Otherwise, these lesions are sporadic except in patients with known solid malignancies and leukemia and lymphoma that may have CVN and CPA involvement.
Prevalence and Epidemiology
CVN and CPA (vestibular) schwannomas account for the vast majority of CPA and CVN tumors. Entirely extracanalicular CPA neurogenic tumors are unusual. These tumors are typically schwannomas of the CVN that almost exclusively arise from its vestibular division. Theoretically, the CVN schwannomas arise most commonly from or lateral to the developmental zone of transition of central myelin to peripheral myelin. This zone is generally located in the outer third of the IAC; thus, early vestibular schwannomas will often be first visible in that location.
Schwannomas or neurofibromas associated with NF-1 or NF-2 make up a small percentage of these lesions.
Meningiomas of the CPA and affecting the CVN account for fewer than 10% of these tumors. Meningiomas may be associated with NF-2 and mosaics.
Metastases are usually meningeal, either dural or pia-arachnoid, in their pattern. CVN dysfunction is very uncommon as a presenting condition for the responsible primary cancers that produce these metastases. When this occurs, facial palsy usually accompanies the CVN deficit early in its course.
Other tumors that present primarily with CVN symptoms are uncommon and sporadic. These include pontine gliomas; ependymomas; and occasionally a skull base lesion such as a paraganglioma, hemangiopericytoma, chondrosarcoma, or chordoma.
Clinical Presentation
Patients will generally present with slowly progressive, asymmetric, high-frequency sensorineural hearing loss; unilateral, continuous, high-pitched tinnitus; and vestibular symptoms in the form of vertigo or balance problems. The hearing loss may be sudden. In patients with large tumors, other cranial nerve dysfunction such as that of the facial and/or trigeminal nerves and symptoms of increased intracranial pressure may occur. Once the tumor compresses the brain stem and cerebellum, ipsilateral upper and lower extremity dysfunction, ataxia, and gait disturbances will develop. Spontaneous or positional provoked nystagmus may be present on physical examination. Larger tumors can cause trigeminal nerve sensory deficits including a diminished corneal reflex.
Audiometry will show an asymmetric hearing loss with speech discrimination that is disproportionately poor. Brain stem evoked response (BSER) testing will often provide a retrocochlear localization of the problem by delayed latencies or poor waveforms.
Pathophysiology and Patterns of Disease
These lesions will compress or invade the CVN, thus interfering with its function. Sudden hearing loss is thought to be the result of occlusion of the internal auditory artery, arising from the anterior inferior cerebellar artery or basilar artery. When large enough, they might cause the same problems with the facial nerve. Very large tumors may compress the lower cranial nerves, resulting in a more global posterior fossa condition. More discreet benign lesions will grow typically as a focal elliptical or spherical mass at a rate of 1 to 3 mm or less per year. In older individuals, a schwannoma may actually shrink with age. Other lesions will grow longitudinally along the nerve to invade/involve the inner ear or petrous portion of the temporal bone.6–13
The natural history of the more common tumors arising in this location and their relationship to the phacomatoses is discussed in the following chapters:
- Nerve sheath tumors (Chapter 29)
- Meningiomas (Chapter 31)
- Chondrosarcomas and chordomas (Chapters 34 and 39)
- Lymphoma and leukemia (Chapters 27 and 28)
- Metastases (Chapter 42)
- Perineural spread of malignancies (Chapter 21 and 22)
- Lipoma (Chapter 36 and Fig. 134.2)
Manifestations and Findings
Computed Tomography and Magnetic Resonance
The MR and CT appearance and growth patterns of the tumors arising in this location are discussed and shown in the following chapters and figures:
- Nerve sheath tumors (Chapter 29). Schwannomas and neurofibromas will generally grow in a vector associated with the CVN (Figs. 29.1 and 29.27A–D).
- The classic growth pattern is that of a mass involving both the IAC and CPA (Fig. 134.3). Less frequently, nerve sheath tumors are confined to either the CPA (Fig. 134.4) or the IAC (Fig. 134.5). Infralabyrinthine tumors are rare (Fig. 134.6). The imaging appearance is variable and depends on the size and histologic features of the lesion (Figs. 134.3–134.7).
- Meningioma (Chapter 31). A meningioma will generally grow with at least some dural vector component (Figs. 31.15 and 134.8).
- Lipoma (Chapter 36). A lipoma is primarily located in the CPA and grows extremely slowly, thereby infiltrating cranial nerves (Figs. 130.7 and 134.2).
- Chondrosarcoma and chordoma (Chapters 39 and 34). These tumors will involve the central skull base and petrous apex in some manner (Fig. 134.9).
- Lymphoma and leukemia (Chapters 27 and 28). These cellular infiltrations and accumulations will generally show a pia-arachnoid, dural, or mixed meningeal spread pattern but may aggregate into a mass (Figs. 27.15E–H and 131.3).
- Metastases (Chapter 42). Extra-axial metastases will most often show a pia-arachnoid, dural, or mixed meningeal (meningeal carcinomatosis) spread pattern (Fig. 134.10). Metastases involving the CPA are mostly seen in lung or breast cancer and melanoma. Occasionally, primary melanoma may arise from melanocytes in the meninges.14
- Perineural spread of malignancies (Chapters 21 and 22). Perineural spread will enlarge the nerve affected or produce a meningeal carcinomatosis pattern (Fig. 131.3).

FIGURE 134.2. Lipoma of the cerebellopontine angle (CPA). A, B: A small lesion with fat density is seen in the right CPA (arrows) and possibly in the internal auditory canal (arrowhead in A).



FIGURE 134.3. Magnetic resonance appearance and growth pattern of a vestibular schwannoma. The classical appearance is illustrated in several patients. A–C: Patient 1. Vestibular schwannomas arise from the inferior or superior vestibular nerve, usually in the outer third of the internal auditory canal, as shown in a three-dimensional (3D) Steady State (SS) in image (A), a parasagittal reconstruction of (A) in image (B), and contrast-enhanced T1 weighting in image (C). D: Patient 2. A vestibular schwannoma will grow toward the porus and cerebellopontine angle (CPA), becoming slightly lobulated with enlargement as can be seen on this 3D SS image. E: Patient 3. Once a vestibular schwannoma grows into the CPA, it tends to show the typical “ice cream on cone” appearance. F–I: Patients 4 and 5. With continued growth, mainly of the extrameatal component of a schwannoma, the histology changes to a predominance of Antoni type B cells, and cystic regression and necrosis will cause a cystic appearance (F) or inhomogeneous enhancement (H). Unusually rapid growth in both of these patients after 1 (G) and 2 (I) years, respectively, illustrates the progressive cystic/necrotic changes with increasing volume. (NOTE: Such rapid enlargement tends to occur in younger patients, and part of it can be due to intratumoral bleeding.) J–N: Patients 6 and 7. Large vestibular schwannomas may cause mass effect and arachnoid cyst formation. In (J) and (K), contrast-enhanced T1-weighted images show that a partially cystic CPA mass is compressing cranial nerve V, the brain stem, and the cerebellum and likely is producing an arachnoid cyst (arrow in J). Note also a cavernous meningioma on the right. In (L), the 3D SS image shows tumoral cystic/necrotic parts (arrowheads) and possible zones of old hemorrhage and fibrosis (white arrow). There is a suspicion of arachnoid cyst formation due to pseudoduplication of the arachnoid by peritumoral adhesions posterior to the mass (black arrow). In (M) and (N), in patient 7 it is difficult to tell a tumoral cyst from a secondary arachnoid cyst (black arrows) on the T2-weighted image in (M), although there may be mass effect on a vessel (black arrowhead) while the solid part of the tumor is clearly delineated (white arrow). In (N), the enhancing wall identifies the more anterior tumoral cyst (arrow), while the arachnoid cyst wall (arrowhead) does not enhance.


FIGURE 134.4. Magnetic resonance appearance and growth pattern of an extracanalicular vestibular schwannoma. Patients become symptomatic by compression of the cerebellum, brain stem, and fourth ventricle rather than from hearing loss. Thus tumors are usually large at presentation. Acute presentations and deterioration clinically in these patients can be due to bleeding with acutely worsening brain stem compression and/or acute obstructive hydrocephalus. A, B: Patient 1. Purely extracanalicular lesions always arise in close proximity to the meatus and are therefore centered on the porus. This patient developed subacute hydrocephalus that can be life threatening. C: Patient 2 likely deteriorated due to subacute intratumoral bleeding (arrows). D–F: Patient 3 had a smaller extracanalicular lesion but presented with acute hearing loss, with the deterioration possibly due to bleeding. In (D), the non–contrast-enhanced T1-weighted (T1W) image shows possible subacute blood products. In (E), the contrast-enhanced T1W image shows typical enhancement but more prominent flow voids than usual. In (F), the T2-weighted image shows very prominent vascularity, which might explain the tendency to intratumoral bleeding but also raises the possibility that this is some other type of lesion, such as a vascular metastasis. This proved to be a vestibular schwannoma at surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree