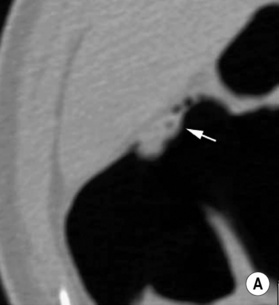
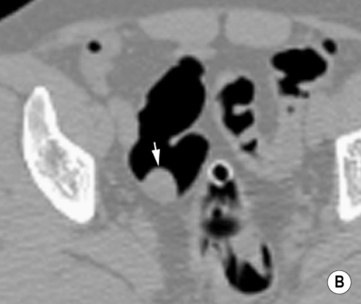
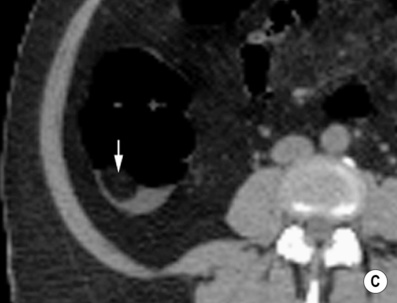
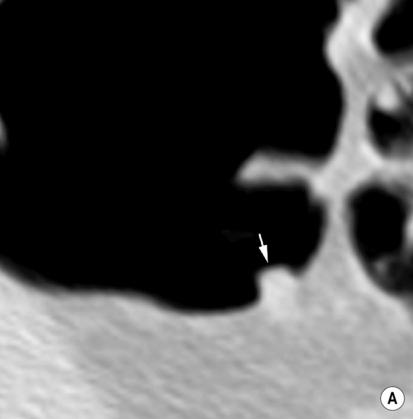
Double-contrast barium enema (DCBE) • Crypt abscesses: may erode through the muscularis mucosae and spread laterally within the submucosa: • Ruptured crypt abscesses lead to superficial erosions which fill with barium to produce a typical granular mucosal pattern (producing continuous ulceration on a background of diffusely abnormal mucosa – discrete ulceration with normal intervening mucosa is not seen) • Reflux ileitis: there is a patulous ileocaecal valve and a granular distal ileum • Postinflammatory polyps: when an acute attack remits, the granulation tissue forming at the ulcer base undermines the residual oedematous mucosal flap at the ulcer edge – this is therefore prevented from sealing down, resulting in sessile, filiform, frond-like polyps (less commonly found in Crohn’s disease) • Chronic colitis: a tubular, shortened, featureless (‘lead-pipe’) colon • Strictures: chronic hypertrophy of the muscularis mucosa (and submucosal thickening with fat) can cause generalized colonic shortening as well as localized left-sided colonic strictures (10–20%) • Acute disease: wall thickening (≥ 4mm) tending to be less marked than with Crohn’s disease • Chronic disease: a widened presacral space due to fibrofatty proliferation • ‘Target’ sign: due to chronic muscularis mucosae thickening and fatty submucosal infiltration (visible even with NECT) • There is an increased risk of colorectal carcinoma (due to dysplastic changes within diseased epithelium rather than from a prior adenoma) – this is more common with an extensive colitis of > 10 years duration • A fulminating colitis: transmural inflammation and ulceration extends deep into the muscular layers with neuromuscular degeneration • It can also occur in any other cause of colitis but is less frequently seen in Crohn’s disease, bacterial colitis, pseudomembranous colitis or ischaemic colitis • It usually affects the transverse colon (the least dependent part of the colon where intraluminal gas collects) • A daily plain AXR is important for assessing and monitoring the colitis extent (barium studies are contraindicated due to the perforation risk) Distinguishing features between ulcerative and Crohn’s colitis* • Mild disease: initially the mucosa is the most susceptible to vascular compromise (with oedema, haemorrhage, or necrosis) • Severe disease: necrosis of the submucosal and muscle layers leads to fibrosis and stricture formation • Salmonella: there may be a marked ileus during the acute stage • Shigella: this usually affects the sigmoid colon (with aphthoid-type ulceration) • Campylobacter: this affects the distal colon • Gonococcus: this usually affects the rectum • Amoebiasis: this usually affects the right colon and caecum • Strongyloides stercoralis: this may simulate UC • Chagas’ disease: a megacolon results from the neurotoxic effect of the protozoon Trypanosoma cruzi • Schistosomiasis: ova are deposited within the large bowel submucosa • This is defined as a circumscribed area of dyplastic epithelium (an intraepithelial neoplasia) • An adenoma can be tubular (65%), tubulovillous (25%) or villous (10%) in nature • Location: rectosigmoid colon (60%) • Size is the most important single indicator for the likelihood of malignancy: • Localized areas of increased attenuation (the incident X-ray beam passes through more than 2 barium layers and consequently less gas) • A thin layer of contrast medium covers the mucosa, forming a ring around the polyp base • Pedunculated polyps: the axis of the stalk usually runs obliquely to the lumen axis (making it easy to distinguish from a haustral fold) • Juvenile polyps: these are smooth and pedunculated with a thin stalk (affecting patients <40 years old) • Postinflammatory polyps: these have a filiform configuration (i.e. finger-like submucosal projections covered by mucosa on all sides) • Villous polyps: these demonstrate a lace-like or mosaic appearance as barium fills the tumour interstices • CTC is equivalent to colonoscopy in the detection of polyps >7mm in size • Technique: full bowel preparation is standard, although reduced preparation regimens with faecal tagging are being developed • Distinguishing a polyp from faecal residue: • An autosomal dominant condition (caused by an APC tumour suppression gene mutation on chromosome 5q21)
Colon
ULCERATIVE COLITIS AND TOXIC MEGACOLON
ULCERATIVE COLITIS (UC)
Radiological features
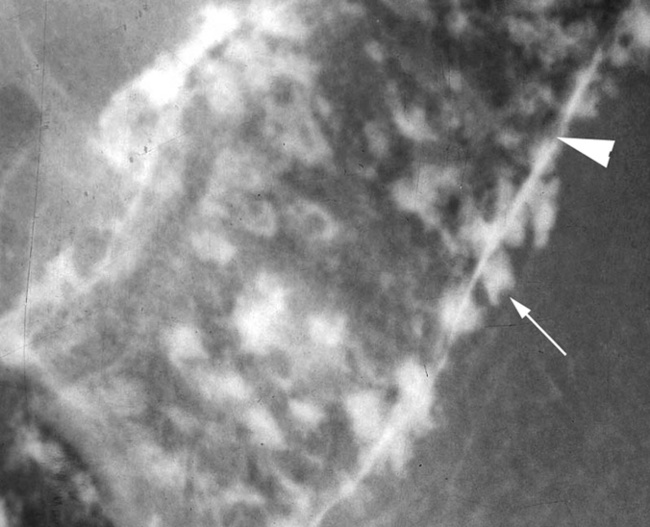
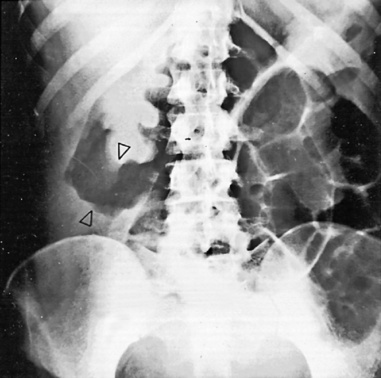
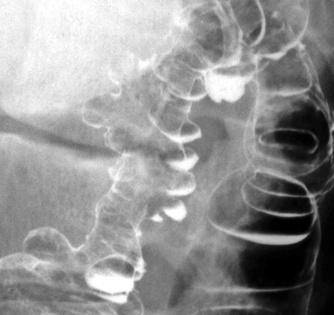
 En face appearance: linear, transverse, serpiginous or rounded
En face appearance: linear, transverse, serpiginous or rounded
 Tangential appearance: undercutting of the mucosal edge can give a ‘T’ or ‘collar stud’ shape
Tangential appearance: undercutting of the mucosal edge can give a ‘T’ or ‘collar stud’ shape
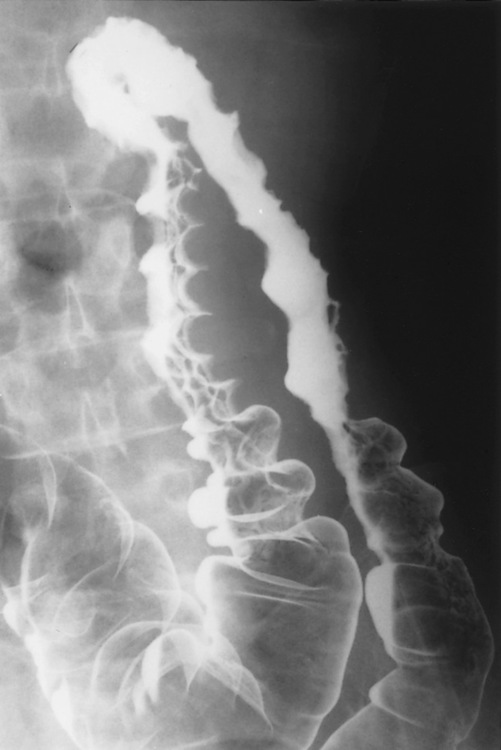
 the strictures are smooth, tapering and symmetrical (cf. asymmetrical strictures in Crohn’s disease)
the strictures are smooth, tapering and symmetrical (cf. asymmetrical strictures in Crohn’s disease)
 absence of formed faecal residue within any affected segments
absence of formed faecal residue within any affected segments  normal pericolonic tissues (unless perforation has occurred)
normal pericolonic tissues (unless perforation has occurred)
Pearls
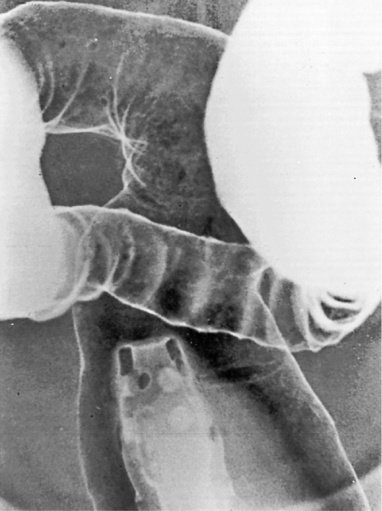
 tumours are frequently multiple and infiltrative
tumours are frequently multiple and infiltrative
 Dysplasia-associated lesions (DALMs): representing severe dysplasia and are a very high risk marker for cancer (similar to a villous adenoma)
Dysplasia-associated lesions (DALMs): representing severe dysplasia and are a very high risk marker for cancer (similar to a villous adenoma)
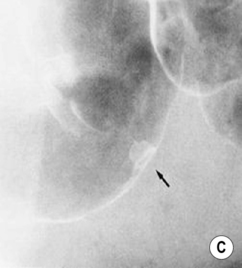
 Early infiltrative carcinoma: this presents with a fixed, irregular, in-drawn base
Early infiltrative carcinoma: this presents with a fixed, irregular, in-drawn base
 Strictures: these are usually benign
Strictures: these are usually benign  malignancy is suggested by an irregular raised area, shouldering or asymmetry
malignancy is suggested by an irregular raised area, shouldering or asymmetry
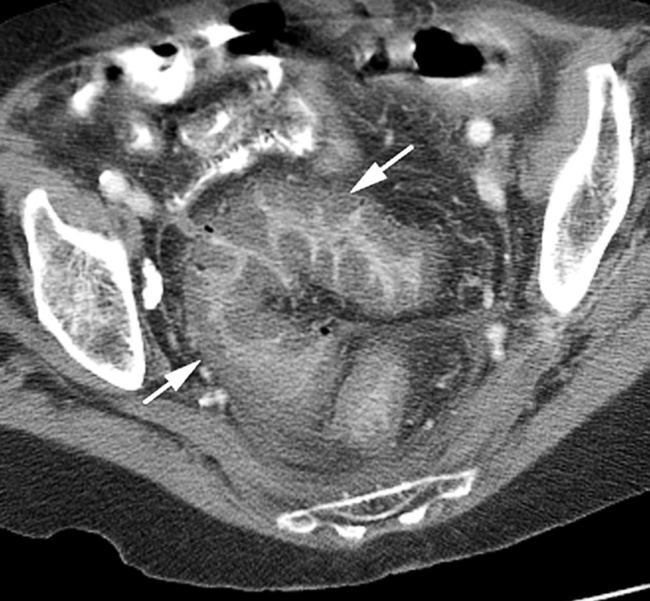
TOXIC MEGACOLON
Definition
 it accounts for most UC-related deaths
it accounts for most UC-related deaths
Radiological features
 perforation is frequent
perforation is frequent
 Dilatation: if > 5cm it is associated with deep ulceration into the muscular layers (> 8.5cm in established cases)
Dilatation: if > 5cm it is associated with deep ulceration into the muscular layers (> 8.5cm in established cases)  the haustra are always absent
the haustra are always absent
Radiographic feature
Ulcerative colitis
Crohn’s disease
SB involvement
Reflux ileitis only
+++
Rectal involvement
Always
50%
Multiple anal fistula
−
+
Aphthoid ulceration
−
+++
Fissuring ulceration
−
++
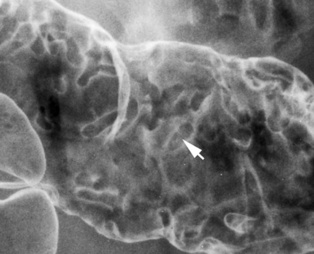
Granularity
+++
+
Transverse symmetry
Symmetric
Asymmetric
Longitudinal extent
In continuity
Discontinuous
Free perforation
+
−
Toxic megacolon
+
−/+
Cancer risk
+
−/+
Entero-enteric fistula
−
+
Submucosal inflammation
++ in chronic disease
−
Mesenteric inflammation
−/+
++
Enlarged lymph nodes
−
+
Fibrofatty proliferation
Mesorectal only
++
COLITIS
ISCHAEMIC COLITIS
Definition
 recovery is usually complete
recovery is usually complete
 transmural necrosis is life-threatening (due to the risk of perforation)
transmural necrosis is life-threatening (due to the risk of perforation)
INFECTIOUS COLITIS
 a toxic megacolon has been reported
a toxic megacolon has been reported
 It leads to a segmental or diffuse colitis with granular or ulcerated mucosa (± aphthoid-type ulceration)
It leads to a segmental or diffuse colitis with granular or ulcerated mucosa (± aphthoid-type ulceration)
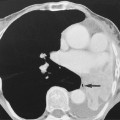
 An amoeboma (an inflammatory granulation mass) is seen in 10% of cases – it can cause irregular stricturing (mimicking a carcinoma)
An amoeboma (an inflammatory granulation mass) is seen in 10% of cases – it can cause irregular stricturing (mimicking a carcinoma)
 Disease is limited to the caecum in 3% of cases, producing a characteristic conical caecum and a shaggy ulcerated mucosa
Disease is limited to the caecum in 3% of cases, producing a characteristic conical caecum and a shaggy ulcerated mucosa  it may be complicated by appendicitis
it may be complicated by appendicitis
 Embolic liver spread is seen in 15% of cases
Embolic liver spread is seen in 15% of cases
 Cytomegalovirus: this demonstrates an ileocolic distribution
Cytomegalovirus: this demonstrates an ileocolic distribution  there is a thick-walled vasculitis with large bleeding ulcers
there is a thick-walled vasculitis with large bleeding ulcers  mesenteric adenopathy and often ascites is present
mesenteric adenopathy and often ascites is present
 Herpes simplex virus: this leads to a proctitis with multiple superficial ulcers
Herpes simplex virus: this leads to a proctitis with multiple superficial ulcers
 Chlamydia trachomatis: this causes lymphogranuloma venereum, which is a chronic proctitis complicated by fistula formation, extensive fibrosis and eventual stricturing
Chlamydia trachomatis: this causes lymphogranuloma venereum, which is a chronic proctitis complicated by fistula formation, extensive fibrosis and eventual stricturing
PARASITIC COLITIS
 the inflammatory response results in polyp formation
the inflammatory response results in polyp formation  fibrosis may later cause stricture formation (± bowel wall calcification)
fibrosis may later cause stricture formation (± bowel wall calcification)
POLYPS
POLYPS
DEFINITION
TYPES
Epithelial
 they are found in 25% of the population over 50 years old (they are rare in patients under 30 years of age)
they are found in 25% of the population over 50 years old (they are rare in patients under 30 years of age)
 villous adenomas may present with electrolyte disturbances due to excessive mucus production
villous adenomas may present with electrolyte disturbances due to excessive mucus production
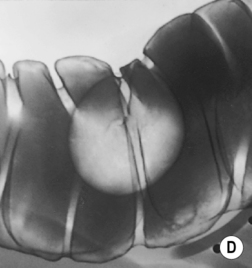
 Pedunculated adenoma: extrusion of a stalk of mucosa and muscularis mucosae may occur as the adenoma is pulled by the faecal stream
Pedunculated adenoma: extrusion of a stalk of mucosa and muscularis mucosae may occur as the adenoma is pulled by the faecal stream
 Sessile adenoma: a broad-based lesion (the base must be at least twice that of its height)
Sessile adenoma: a broad-based lesion (the base must be at least twice that of its height)
 Flat adenoma: a lesion with a height that is no more than twice the height of the adjacent normal mucosa
Flat adenoma: a lesion with a height that is no more than twice the height of the adjacent normal mucosa  it is categorized into a slightly elevated, completely flat, or slightly depressed lesion
it is categorized into a slightly elevated, completely flat, or slightly depressed lesion
 descending colon (18%)
descending colon (18%)  transverse colon (14%)
transverse colon (14%)  ascending colon and caecum (8%)
ascending colon and caecum (8%)
 Adenomas tends to be larger when present within the left colon (⅔ of polyps > 2cm are within the rectosigmoid colon)
Adenomas tends to be larger when present within the left colon (⅔ of polyps > 2cm are within the rectosigmoid colon)
RADIOLOGICAL FEATURES
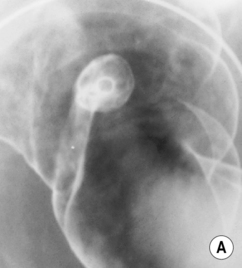
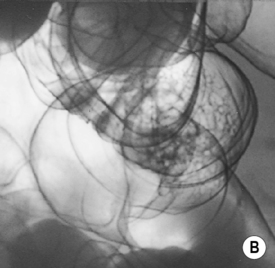
DCBE appearances of polyps
 Viewed en face: this creates a ring shadow with a sharp inner border and an outer margin fading into the normal surface coating (cf. the opposite appearance with an ulcer)
Viewed en face: this creates a ring shadow with a sharp inner border and an outer margin fading into the normal surface coating (cf. the opposite appearance with an ulcer)
 some may present as a flat, nodular, carpet-like growth (with minimal elevation) within the rectosigmoid colon or caecum
some may present as a flat, nodular, carpet-like growth (with minimal elevation) within the rectosigmoid colon or caecum
CT colonography (CTC) appearances of polyps
 CO2 distension is used (and improved with IV Buscopan)
CO2 distension is used (and improved with IV Buscopan)  supine and prone sequences are reviewed to reduce the chance of any collapsed or fluid-filled segments hiding a lesion
supine and prone sequences are reviewed to reduce the chance of any collapsed or fluid-filled segments hiding a lesion  the original datasets and 3D reformatted images (which are useful for problem solving) are reviewed
the original datasets and 3D reformatted images (which are useful for problem solving) are reviewed
 Pitfall: an inverted diverticulum may simulate a polyp on 3D images (on 2D images it will contain gas)
Pitfall: an inverted diverticulum may simulate a polyp on 3D images (on 2D images it will contain gas)
POLYPOSIS SYNDROMES
FAMILIAL ADENOMATOUS POLYPOSIS (FAP)
DEFINITION
 it is characterized by multiple (500–2500) colonic adenomas and requires at least 100 adenomas to be present for the diagnosis to be made
it is characterized by multiple (500–2500) colonic adenomas and requires at least 100 adenomas to be present for the diagnosis to be made
 The polyps develop by the early teens – all patients will eventually develop colorectal cancer (accounting for 1% of all colorectal cancers)
The polyps develop by the early teens – all patients will eventually develop colorectal cancer (accounting for 1% of all colorectal cancers)  a restorative proctocolectomy is recommended once the condition is diagnosed
a restorative proctocolectomy is recommended once the condition is diagnosed
 Associations: hamartomatous stomach polyps (> 50% of patients)
Associations: hamartomatous stomach polyps (> 50% of patients)  duodenal adenoma (almost 100% of patients)
duodenal adenoma (almost 100% of patients)  periampullary carcinoma (5% of patients)
periampullary carcinoma (5% of patients)
Colon

 there is symmetrical colonic involvement (cf. asymmetrical Crohn’s disease)
there is symmetrical colonic involvement (cf. asymmetrical Crohn’s disease)
 an acute fulminating colitis with a risk of perforation (15%)
an acute fulminating colitis with a risk of perforation (15%)  extracolonic manifestations:
extracolonic manifestations:
 ankylosing spondylitis
ankylosing spondylitis  sacroiliitis
sacroiliitis  erythema nodosum
erythema nodosum  pyoderma gangrenosum
pyoderma gangrenosum  primary sclerosing cholangitis
primary sclerosing cholangitis  cholangiocarcinoma
cholangiocarcinoma  iritis
iritis a stratified appearance with differentiation between the submucosa and muscularis propria
a stratified appearance with differentiation between the submucosa and muscularis propria  ulceration (with focal disruption of the bowel wall layers which may be outlined by intracolonic gas)
ulceration (with focal disruption of the bowel wall layers which may be outlined by intracolonic gas)  inflammatory echogenic pericolic fat
inflammatory echogenic pericolic fat perforation
perforation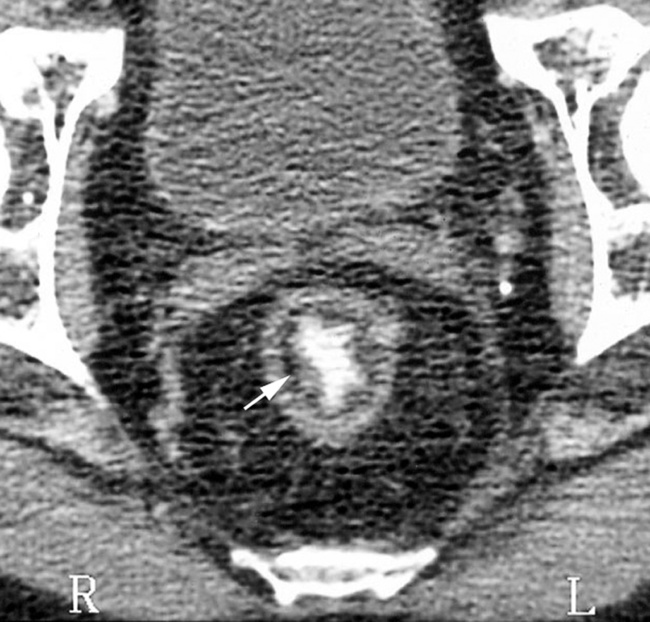
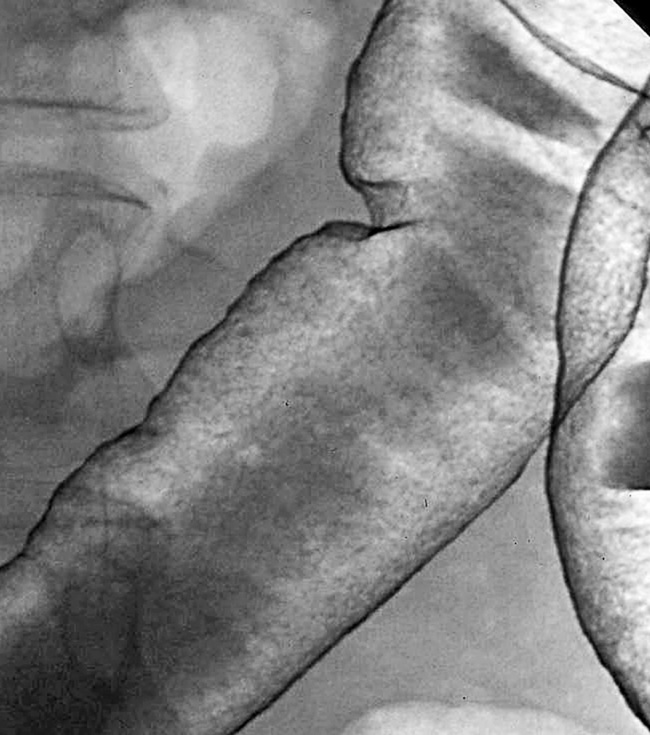
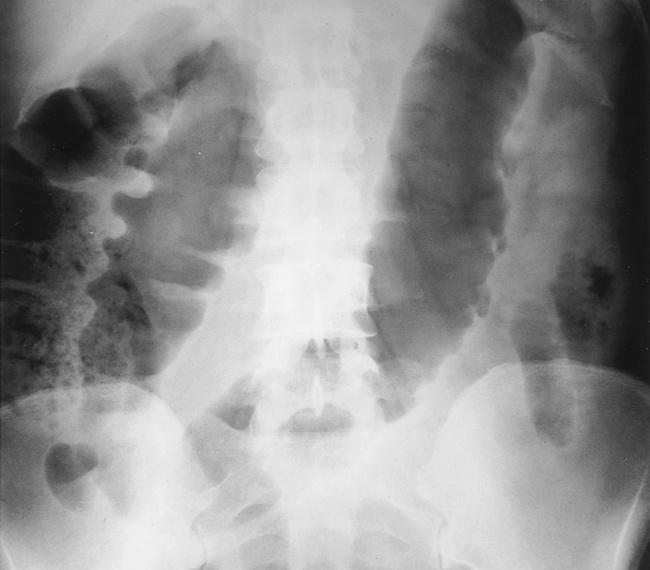
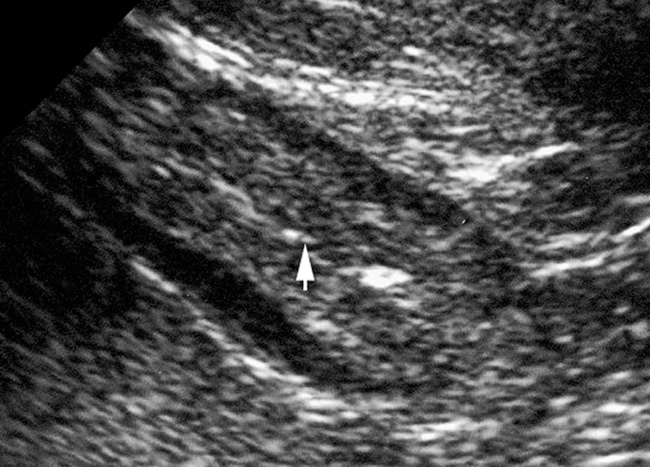
 it is associated with bacterial superinfection
it is associated with bacterial superinfection it often requires surgery
it often requires surgery it can be treated conservatively
it can be treated conservatively mucosal thumb-printing (due to submucosal haemorrhage and oedema)
mucosal thumb-printing (due to submucosal haemorrhage and oedema)  free air (secondary to perforation)
free air (secondary to perforation)  colonic sacculation
colonic sacculation submucosal oedema can cause a ‘target’ sign
submucosal oedema can cause a ‘target’ sign free fluid
free fluid  pneumatosis
pneumatosis  portomesenteric gas
portomesenteric gas there can be a ‘slit-like’ IVC (as a result of the low intravascular volume)
there can be a ‘slit-like’ IVC (as a result of the low intravascular volume) it is a late complication (often presenting years after radiotherapy when the total dose is > 45Gy)
it is a late complication (often presenting years after radiotherapy when the total dose is > 45Gy) strictures are usually smooth and symmetrical unless there is superimposed ulceration
strictures are usually smooth and symmetrical unless there is superimposed ulceration  fistula formation is commonly to the bladder or vagina
fistula formation is commonly to the bladder or vagina  perforation is rare
perforation is rare increased mesorectal fat and a thickened mesorectal fascia
increased mesorectal fat and a thickened mesorectal fascia  a widened presacral space
a widened presacral space pneumatosis
pneumatosis  mesenteric stranding and small bowel involvement is common
mesenteric stranding and small bowel involvement is common it is usually as a result of broad-spectrum antibiotic therapy and may be life-threatening
it is usually as a result of broad-spectrum antibiotic therapy and may be life-threatening mucosal thumbprinting
mucosal thumbprinting ascites
ascites  small bowel dilatation
small bowel dilatation  marked mucosal enhancement
marked mucosal enhancement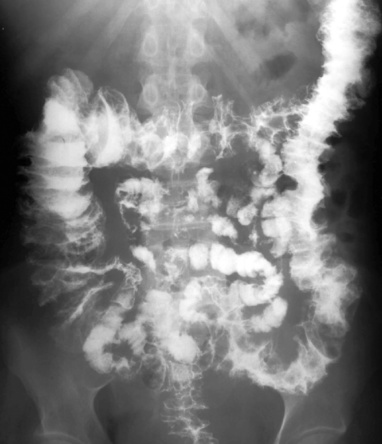

 1–2cm (10% risk)
1–2cm (10% risk)  >2cm (approximately a 50% risk)
>2cm (approximately a 50% risk)
 they may demonstrate a more rapid progression to overt cancer than a polypoid tumour
they may demonstrate a more rapid progression to overt cancer than a polypoid tumour leiomyoma
leiomyoma  lipoma
lipoma  fibroma
fibroma it is usually a solitary right colonic lesion (and may cause pain, bleeding or intussusception if > 4cm)
it is usually a solitary right colonic lesion (and may cause pain, bleeding or intussusception if > 4cm)
 they are common within the rectum
they are common within the rectum  there is no malignant potential unless they demonstrate a ‘serrated’ appearance
there is no malignant potential unless they demonstrate a ‘serrated’ appearance



 faecal residue often contains internal gas locules
faecal residue often contains internal gas locules