Colorectal Cancer
Todd M. Blodgett, MD
Alex Ryan, MD
Omar Almusa, MD
Key Facts
Imaging Findings
PET poorly sensitive for small (< 1 cm) lesions; high positive predictive value
PET insensitive (29%) for small (< 1 cm) regional lymph nodes
Clinical management: PET affects surgical planning in approximately 30% of colorectal cancer patients
PET/CT more sensitive for regional/distant metastases than CT alone
> 95% sensitivity and ˜ 71% specificity for localization of relapse in patients with increased CEA
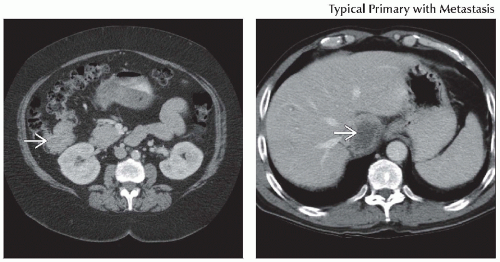
Staging: PET/CT will often show additional lesions not seen on CT, particularly in the liver
Restaging: Combination of a rising CEA level and a focal abnormality on PET/CT, often without a correlative CT abnormality
May be focal at ileocecal valve
Top Differential Diagnoses
Adenomas
Abscess
Physiologic FDG activity in bowel
Clinical Issues
Recurrence: Rising CEA level, abdominal pain (obstruction)
Diagnostic Checklist
Pre-treatment PET for staging, confirmation of FDG-avid disease
Mucinous adenocarcinoma has variable PET activity
Correlate focal increased activity in bowel on FDG PET with colonoscopy
TERMINOLOGY
Abbreviations and Synonyms
Colorectal carcinoma (CRC), colon cancer, adenocarcinoma of the colon, rectal carcinoma
Definitions
Malignancy of the colon &/or rectum
IMAGING FINDINGS
General Features
Best diagnostic clue
Initial diagnosis: None, usually indicated in history, although incidental focal intense FDG activity may represent an incidental malignant lesion
Staging: PET/CT will often show additional lesions not seen on CT, particularly in the liver
Restaging: Combination of a rising CEA level and a focal abnormality on PET/CT, often without a correlative CT abnormality
Location
Initial diagnosis: Colon/rectum
Staging: Additional liver lesions will often affect patient management
Recurrence: Surgical anastomosis, regional lymph nodes, presacral area
Size: Variable, although PET has poor performance with small lesions including carcinomatosis
CMS coverage 2009: Initial diagnosis, staging, restaging; response to therapy not currently covered
Nuclear Medicine Findings
General
Physiologic FDG activity
Very common
Range from no activity to intense
Usually linear in appearance
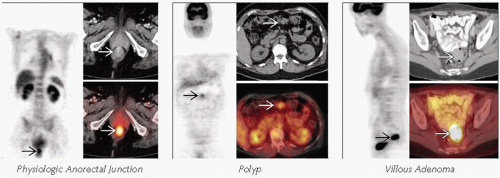
Right colon and cecum more commonly demonstrate increased physiologic FDG activity
May be focal at ileocecal valve
Short segment or linear FDG activity in bowel without correlative CT abnormality almost always physiologic
Focal or short segment moderate to intense activity is also common at the anorectal junction
PET poorly sensitive for small (< 1 cm) lesions; however, high positive predictive value
PET has limited sensitivity for peritoneal, omental metastases, highly mucinous tumors (may not be FDG avid)
Positive predictive value high for detection of omental or peritoneal disease, which may be difficult to detect with CT alone
Primary colon cancers may be incidentally identified with PET
Focal activity on FDG PET should be followed subsequently evaluated with colonoscopy
1-3% of patients undergoing PET/CT will have incidental accumulation in GI tract
Associated with substantial risk of underlying cancer or pre-cancerous lesion
Initial diagnosis
Not recommended for screening, but PET/CT may play a role in screening patients with familial polyposis
PET and PET/CT are CMS-covered but rarely used for initial diagnosis of colon cancer
Colonoscopy is the preferred method for initial diagnosis
Colonic adenoma and benign polyps can take up significant FDG and appear similar to carcinoma
Not used for diagnosis of polyps &/or adenomas
However, FDG PET has 84% specificity for detecting colonic adenomas
Specificity improves with increasing size and grade of adenoma
Staging
Clinical management: PET affects surgical planning in approximately 30% of colorectal cancer patients
Main indication for PET/CT in CRC is assessment for consideration for metastasectomy
Goal of avoiding major surgery in patients with undetected nodal/distant metastases
Consider pre-treatment PET for staging of all high risk patients and confirmation of FDG-avid disease
PET insensitive (29%) for small (< 1 cm) regional lymph nodes
Colon metastases most commonly go to liver
Accuracy of distant staging for colorectal cancer: PET 78%, PET/CT 89%
PET/CT more sensitive for regional/distant metastases than CT alone
Rectal metastases may bypass liver and metastasize to lung
Inspection of CT is pertinent to detect small pulmonary nodules that may be missed on PET
Mucinous adenocarcinoma metastases may show calcification on CT
May also be falsely negative on PET
Consider staging PET or PET/CT in any patient with a high risk primary lesion (> Dukes A lesion at surgery)
Restaging
Established role for PET/CT in patients with suspected recurrent disease, particularly in patients with rising CEA levels
Restage to detect locally recurrent disease, isolated metastatic disease in liver/lung, diffuse metastases
> 95% sensitivity and ˜ 71% specificity for localization of relapse in patients with increased CEA
PET to differentiate scar/fibrosis after surgery or radiation from tumor in rectal canal
No evidence to support use of PET in routine surveillance following curative primary surgery
Response to chemotherapy
Not currently a CMS-covered indication
Early, i.e., metabolic but not anatomic, response to therapy can be imaged with FDG PET
Also can identify those with biologically aggressive disease unsuitable for resection
Reduction in SUV after 1-3 cycles of chemotherapy may predict response and correlate with subsequent tumor shrinkage
Future chemotherapy that achieves cytostasis over cytotoxicity may benefit from PET imaging
CT Findings
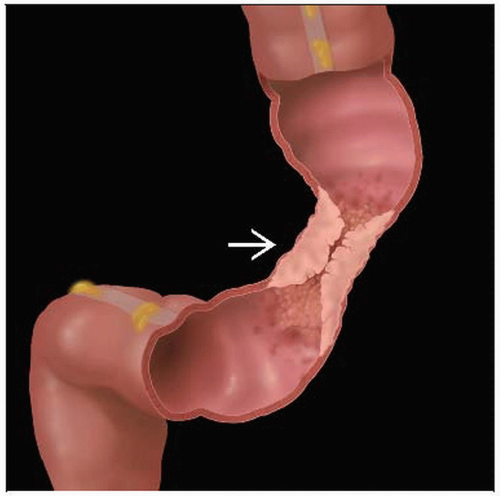
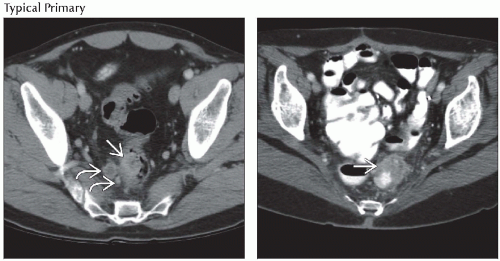
Localized tumor may be seen as intraluminal or intramural mass of soft tissue density adjacent to gasfilled or contrast-filled bowel lumen
No mural thickening or pericolic fat in stage A tumors
Some smaller primary tumors may not be visible on CT or PET
More advanced tumors associated with > 6 mm thickening of bowel wall and infiltration of pericolic fat
Annular carcinoma seen as a thickening of bowel wall and narrowing of lumen
Thickening is concentric given perpendicular scanning plane
Extracolonic tumor spread indicated by loss of tissue fat planes between colon and surrounding structures
Invaded muscle may be enlarged
Colonic tumors may invade anterior abdominal wall, liver, pancreas, spleen, or stomach
Intussuscepting tumor may have target-like appearance with alternating rings of soft tissue and fat on CT
Only seen if mesenteric fat is present between intussusceptum and intussuscipiens
60% of affected lymph nodes are detected by CT
Rectosigmoid tumors may metastasize to external iliac nodes
Liver is most common site of metastasis
CECT shows well-defined areas of low density (relative to normal parenchyma) in portal venous phase
In earlier arterial phase, hepatic mets may show rim enhancement or become hyper-/isodense to normal liver
Other common sites of metastasis are lungs, adrenal glands, peritoneum, and omentum
Adrenal mets may be seen in as many as 14% of patients with CRC
Typical findings include enlargement (> 2 cm), asymmetry, and heterogeneity
Bony and cerebral mets are uncommon
Imaging Recommendations
Best imaging tool
PET/CT for initial staging and restaging
Other modalities: For detection of primary lesion
Colonoscopy, double contrast barium enema (low sensitivity for polyps < 1 cm), and virtual colonography (gaining acceptance)
Imaging of liver
PET/CT for high risk patients
Improves therapeutic management of patients with liver metastases
MR, US for indeterminate cases
Rectal cancer
PET/CT has significant impact on course of treatment through more accurate staging
MR is also established in staging by facilitating accurate assessment of mesorectal fascia
Protocol advice
PET/CT perform with diagnostic rather than noncontrast CT
Will help with abdominopelvic lesions adjacent to bowel and also increase confidence level for confirming hepatic lesions
DIFFERENTIAL DIAGNOSIS
Adenomas
Variable PET activity
Benign adenomas can show intense FDG activity and mimic carcinoma
Inflammatory Bowel Disease
Ulcerative colitis, Crohn disease
Increased activity often seen in affected bowel on PET
Infection
Increased activity in affected segments of bowel
Example: Pseudomembranous colitis
Abscess
Abscess and tumor can both show increased FDG activity
Increased FDG activity surrounding photopenic center = abscess, necrotic tumor
Time course, prior studies useful to differentiate
Gas + fluid collection more specific for abscess
Physiologic FDG Activity in Bowel
Diffuse activity in part of/or throughout bowel
Usually linear
No corresponding bowel thickening on CT
Seroma
Photopenia on FDG PET; fluid density on CT
Post-Radiation Change
Early: Often difficult to assess due to increased FDG activity secondary to inflammation
Typically resolves in 2-6 months
Post-Surgical Scar/Fibrosis
Mildly increased FDG activity with normal post-surgical healing
Serial FDG PET: Scar/fibrosis has stable or decreased activity
PATHOLOGY
General Features
General path comments
Colon polyps
10% of all polyps are adenomatous
Increased incidence of carcinoma in villous tumors
Genetics: Some genetic predisposition in familial polyposis syndromes
Etiology
Arises from pre-existing adenomatous polyps in colonic or rectal mucosa
Age, smoking, diet high in fat and cholesterol, inflammatory bowel disease (mostly ulcerative colitis), genetic predisposition
Epidemiology
3rd most common cancer in USA
135,000 new cases per year in USA; 55,000 deaths per year
Lifetime risk in general population: 5.9%
2/3 of colorectal cancers arise in colon, 1/3 in rectum
Staging, Grading, or Classification Criteria
Modified Dukes staging system for colorectal cancer
Dukes A: Carcinoma in situ limited to mucosa or submucosa (T1N0M0)
Dukes B: Cancer that extends into the muscularis (B1), into or through the serosa (B2)
Dukes C: Cancer that extends to regional lymph nodes (T1-4, N1M0)
Dukes D: Modified classification; cancer that has metastasized to distant sites (T1-4, N1-3, M1)
CLINICAL ISSUES
Presentation
Most common signs/symptoms
GI bleed, seen in 60% of patients presenting with colorectal carcinoma
Colonic adenoma presents: 50% with abdominal pain; 35% with bowel habit changes; 30% with occult bleeding
Other signs/symptoms: Recurrence indicated by rising CEA level, abdominal pain (obstruction)
Demographics
Age: Peak 7th decade; risk rises over age 40
Gender: Male preponderance for colon polyps
Natural History & Prognosis
Dukes A: 5 year > 90%
Dukes B: 5 year > 70%
Dukes C: 5 year < 60%
Dukes D: 5 year ˜ 5%
Small studies have shown improved disease-free and overall survival in patients evaluated with FDG PET imaging prior to surgery
Untreated patients with metastatic disease have life expectancy of 6-12 months
> 20% of patients who present with hepatic metastases are resectable, but surgery remains only potentially curative therapy
5 year overall survival following complete resection of isolated liver metastasis is 30-40% with 10 year survival of ˜ 25%
75% of patients who undergo liver metastasis resection experience relapse
Partly due to inaccurate staging with occult extrahepatic metastases that go undetected prior to surgery
Treatment
Surgically curable if detected early
Adjuvant chemotherapy to prolong survival with lymph node positive disease
Rectal adenocarcinomas are sensitive to radiation
Local recurrence: Surgery, chemo ± radiation
Hepatic recurrence: Resection, radiofrequency ablation, hepatic arterial chemotherapy/radiotherapy
Patients with unresectable disease have median survival up to 20 months with non-surgical therapy
Estimation of gross tumor volume in reference to radiotherapy changed significantly in approximately 50% of patients when metabolic imaging was used
DIAGNOSTIC CHECKLIST
Consider
PET/CT with diagnostic CT for staging and for restaging patients which elevated CEA levels
Also consider PET/CT when the differential diagnosis is scar vs. residual or recurrent tumor
Image Interpretation Pearls
Mucinous adenocarcinoma has variable PET activity
Correlate focal increased activity in bowel on FDG PET with colonoscopy
Rectal cancer: FDG PET to differentiate scar/fibrosis after surgery or radiation vs. tumor
SELECTED REFERENCES
1. de Geus-Oei LF et al: Chemotherapy response evaluation with FDG-PET in patients with colorectal cancer. Ann Oncol. 19(2):348-52, 2008
2. Dresel S et al: PET in colorectal cancer. Recent Results Cancer Res. 170:109-24, 2008
3. Fletcher JW et al: Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 49(3):480-508, 2008
4. Geus-Oei LF et al: Predictive and prognostic value of FDG-PET. Cancer Imaging. 8:70-80, 2008
5. Inoue K et al: Diagnosis supporting algorithm for lymph node metastases from colorectal carcinoma on 18F-FDG PET/CT. Ann Nucl Med. 22(1):41-8, 2008
6. Kristiansen C et al: PET/CT and histopathologic response to preoperative chemoradiation therapy in locally advanced rectal cancer. Dis Colon Rectum. 51(1):21-5, 2008
7. Shin SS et al: Preoperative staging of colorectal cancer: CT vs. integrated FDG PET/CT. Abdom Imaging. 33(3):270-7, 2008
8. Sobhani I et al: Early detection of recurrence by 18FDG-PET in the follow-up of patients with colorectal cancer. Br J Cancer. 98(5):875-80, 2008
9. Soyka JD et al: Staging pathways in recurrent colorectal carcinoma: is contrast-enhanced 18F-FDG PET/CT the diagnostic tool of choice? J Nucl Med. 49(3):354-61, 2008
10. Tan MC et al: A prognostic system applicable to patients with resectable liver metastasis from colorectal carcinoma staged by positron emission tomography with [18F]fluoro-2-deoxy-D-glucose: role of primary tumor variables. J Am Coll Surg. 206(5):857-68; discussion 868-9, 2008
11. Tsunoda Y et al: Preoperative diagnosis of lymph node metastases of colorectal cancer by FDG-PET/CT. Jpn J Clin Oncol. 38(5):347-53, 2008
Image Gallery
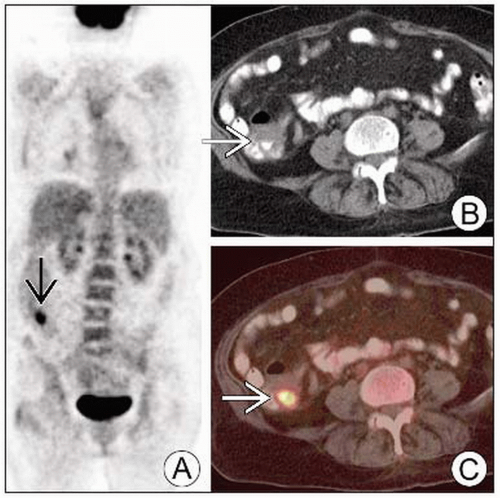
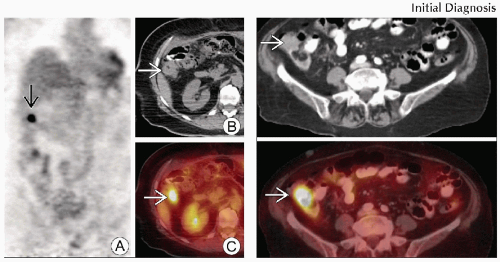 (Left) Coronal PET (A), axial CT (B) and fused PET/CT (C) show a focal area of intense FDG activity in the distal ascending colon
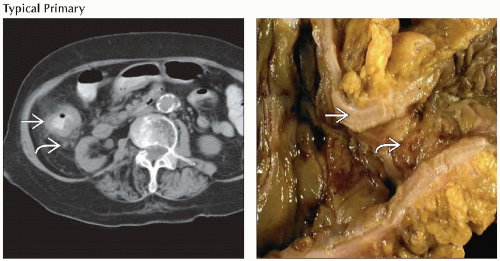
 that corresponds to an area of questionable thickening on the CT portion of the exam that corresponds to an area of questionable thickening on the CT portion of the exam  . (Right) Axial CECT (top) and PET/CT (bottom) show intense FDG activity correlating with a focal mass within the lateral aspect of the proximal ascending colon . (Right) Axial CECT (top) and PET/CT (bottom) show intense FDG activity correlating with a focal mass within the lateral aspect of the proximal ascending colon  . .Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|