Cessation of organ growth
Wrinkling of skin
Loss of teeth
Graying of hair
Atrophy of gonads
Weakening of muscles
Bent posture
Slowed reaction time
Presbyopia
Reduction in rapid eye movement (REM) sleep
Diminution of sense of smell
Loss of fine coordination
Decline of memory and mental efficiency
Loss of hearing, particularly for higher frequencies
Decline in ability to taste, particularly salt and bitter
Diminution of vibratory sense in toes and feet
3 Growth Patterns of Normal Tissues: Influence on Manifestations of Normal Tissue Effects
The growth period for the human body is unusually long among mammalian species, extending for more than a quarter of the normal life span. This long growth period is associated with a delay in most aspects of bodily development, especially skeletal and endocrine maturation. Total body mass continues to increase after maturity, but the rate of increase is slowed considerably after about age 18 years in males and about age 16 years in females.
The growth of specific tissues from birth to adulthood has been categorized by four classic patterns: (1) lymphoid, with accelerated growth followed by involution at the time of puberty; (2) brain, with rapid postnatal growth that slows and essentially completes by early adolescence; (3) gonadal, with little change during early life but rapid development just before and coincident with puberty; and (4) a more general pattern characterized by two major periods of rapid growth during the postnatal period and puberty. Figure 1 shows the growth relationships according to time and type of organ in the neonatal period and childhood (Tanner 1962), while Table 2 provides specific data for a broader number of organs and systems.
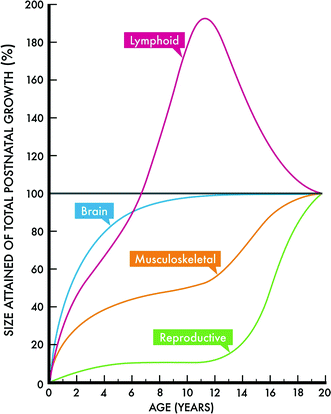

Fig. 1
Four classic growth curves according to age and organ size. Modified with permission from Tanner (1962)
Table 2
Anatomy and physiology: relative rate of development
Age 0–5 years | Age 5–10 years | Age 10–15 years | Age 15–Adult | |
|---|---|---|---|---|
Brain | 4 | 1 | 1 | 1 |
Thyroid | 2 | 3 | 3 | 4 |
GI | 4 | 3 | 3 | 2 |
Gonadal | 1 | 1 | 4 | 4 |
Lung | 3 | 2 | 3 | 4 |
Urinary system | 4 | 3 | 3 | 2 |
Skin | 3 | 2 | 4 | 4 |
Lymphoid tissues | 4 | 4 | hypoplasia | hypoplasia |
Liver | 4 | 2 | 3 | 2 |
Musculoskeletal | 3 | 2 | 4 | 2 |
Head and neck | 4 | 2 | 2 | 1 |
Circulatory | 3 | 2 | 4 | 2 |
In general, one can expect the timing of radiation therapy along the curve to influence the type and severity of possible late effects, with more pronounced and severe complications occurring if radiation is administered during the periods of increased mitotic activity. These growth curves provide a context in which to explore and discuss the incidence and differential responses of children with respect to the normal tissue effects.
3.1 Lymphoid Growth Pattern
This type of growth pattern is characterized by gradual evolution and involution to the time of puberty. An example of an organ that follows this pattern is the thymus. It reaches its greatest relative weight at birth, but its absolute weight continues to increase until the onset of puberty. In fact in the renewal phase of adulthood, it is estimated that the thymus gland is only about 5–10 % of its original size. No significant late effects from RT occur in the lymphoid system. In particular, there are no age-specific effects, perhaps because the inherent radiation sensitivity of lymphoid tissues outweighs any differential that could be observed due to age.
3.2 Brain Growth Pattern
This type of growth pattern is characterized by rapid postnatal growth which slows down and is almost complete in adolescence. Naturally, the head and neck also follow this pattern of growth.
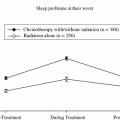
The brain is most sensitive to ionizing radiation in the early fetal period, but postnatally during the first few years of life. Brain growth during the first 3 years of life is not secondary to increase in number of neurons but due to axonal growth, dendritic arborization, and synaptogenesis. Myelinization, though well developed at about the second year of life, is not complete until the second to third decade of life (Dobbing and Sands 1963). By 6 years of age, the child’s brain has reached adult size. Several studies have shown greater cognitive impairment in younger children compared to older children receiving cranial irradiation (Ris et al. 2001; Conklin et al. 2008). It is for this reason that for many years prior to the era of conformal radiotherapy, in an effort to avoid the deleterious effects of radiotherapy, chemotherapy after surgery was the treatment for children <3 years of age with medulloblastoma, ependymoma, and high-grade gliomas (Duffner et al. 1993). A report from St. Jude Children’s Research Hospital showed cognitive decline after conformal radiotherapy for children under 12 years of age with low-grade glioma (Fig. 2). In fact, age at time of irradiation was more important than radiation dose in predicting cognitive decline. Children <5 years old show the most cognitive decline (Merchant et al. 2009). Consistent with this, most children who are affected with radiation-induced Moyamoya syndrome were irradiated when they were <5 years of age, a time when brain growth is rapid (Desai et al. 2006).
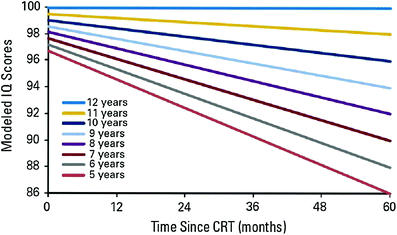

Fig. 2
Intelligence quotient (IQ) scores after conformal radiotherapy in children with low grade glioma. Reprinted with permission from Merchant et al. (2009)
One study attributed a greater degree of deficient development with loss of white matter, and this was presumptively correlated with the impaired cognitive outcome of younger children (Mulhern et al. 2001). This description of white matter changes is consistent with the classic finding after radiation insult of the normal brain of a focal or diffuse area of white matter necrosis (Martins et al. 1977). It is also consistent with the premise that radiotherapy would have more effect during the time when there is greater growth of the brain. Table 3 shows the growth and development of the brain according to time of irradiation.
Table 3
Growth and development of brain with respect to time of irradiation
Phase | Time | Manifestation after radiation therapy |
|---|---|---|
Preimplantation | First 2 weeks post-conception | Death of pre-implanted embryo |
Prenatal organogenesis and fetal period | 2 weeks to parturition | Microcephaly, mental retardation (greatest risk at 8–15 weeks post-conception) |
Neogenesis | Birth to 6 years | Mental retardation, severe cognitive deficits especially in children <3 years of age when myelin formation is still not nearly complete. Hypoplasia of portion of skull and soft tissues which receive radiation therapy |
Genesis | 6 years to puberty | Mild to moderate cognitive deficits. Mild or no hypoplasia of skull as brain reaches its adult size at 6 years of age |
Pubogenesis | Puberty to 18 years | Mild cognitive deficits. No hypoplasia of skull |
Maturation | 18–40 years | Mild cognitive deficits. No hypoplasia of skull |
Renewal and senescence phase | 40 years + | Brain atrophy, cognitive deficits seen but a combination of senescence and radiotherapy effect |
In adulthood, late effects of RT to the brain involve neurocognitive dysfunction which is discussed in greater detail elsewhere in this textbook.
3.3 Gonadal Growth Pattern
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree