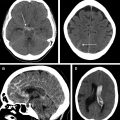
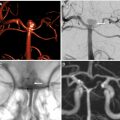
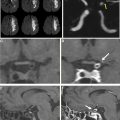
Computed tomography remains the most widely used imaging modality for evaluating patients with acute ischemic stroke. Landmark trials have used computed tomography imaging to select patients for intravenous thrombolysis and endovascular treatment. This review summarizes the most important acute ischemic stroke trials, provides an outlook of ongoing studies, and proposes possible image algorithms for patient selection. Although evaluation with anatomic computed tomography imaging techniques is sufficient in early window patients, more advanced imaging techniques should be used beyond 6 hours from symptoms onset to quantify the ischemic core and evaluate for the salvageable penumbra.
Key points
- •
Multiple randomized, controlled trials have used computed tomography-based imaging to establish efficacy and safety of intravenous thrombolysis and endovascular treatment for acute ischemic stroke.
- •
In the early window (<6 hours), anatomic imaging with noncontrast computed tomography and computed tomography angiography is sufficient to confirm a vascular occlusion and exclude presence of intracranial hemorrhage and a large established infarct.
- •
As the time from symptoms increases beyond 6 hours, more advanced imaging techniques should be used to quantify ischemic core and evaluate for salvageable penumbra.
- •
Several randomized, controlled trials are currently underway that aim to address some of the remaining questions.
- •
Questions include whether endovascular treatment is useful in patients with large infarct cores (>70 mL) or mild stroke symptoms and an extended time window for intravenous thrombolysis.
Introduction
Acute ischemic stroke (AIS) remains a leading cause of disability and death in the United States. There is no doubt that neuroimaging has played a crucial part in the assessment of patients with AIS over the past decades. Although MR imaging is becoming more and more accessible, computed tomography (CT)-based techniques are generally faster to acquire, less costly, and remain most widely used and available.
Evaluation of patients with AIS with CT may include noncontrast CT (NCCT) scan of the head, CT angiography (CTA) of the head (and neck), and/or CT perfusion (CTP) imaging. Together, these modalities can provide information on early ischemic changes and evaluate for concurrent intracranial hemorrhage that may be a contraindication to thrombolysis (NCCT), vascular occlusion, thrombus size, and collateral status (CTA), as well as infarct core and surrounding hypoperfused at-risk brain parenchyma, which is termed the penumbra (CTP). Data from these modalities then allow the treating physician to triage patients to revascularization therapies including intravenous thrombolysis (IVT) and endovascular treatment (EVT), with the goal to identify patients who may benefit from these therapies based on favorable anatomy and physiology.
Initial randomized, controlled trials of IVT and EVT have focus on using imaging to exclude other pathologies with similar clinical presentations (such as intracranial hemorrhage on NCCT) and to exclude a large established infarct that may increase the risk of reperfusion hemorrhage. , Without more nuanced patient selection, however, the benefits of IVT fade at time windows in excess of 4.5 hours, and suboptimal patient selection is one of reasons why several initial trials failed to a show benefit of EVT. It, therefore, seems logical that better patient selection with advanced imaging techniques that assess patient physiology would allow for selection of patients with a greater chance to benefit, even in extended time windows.
Indeed, between 2015 and 2018 several randomized trials were published that demonstrated efficacy of EVT in patients with AIS selected with advanced CT-based imaging techniques, and several additional trials are ongoing. Consequently, the treating physician at this time has a plethora of imaging algorithms for AIS patient selection to choose from that are supported by level I evidence. In this review, we describe the current evidence and possible imaging algorithms for AIS patient selection to both IVT and EVT. Furthermore, we describe the current gaps in level I evidence and ongoing randomized, controlled trials that are aimed to address some of the remaining questions.
Noncontrast computed tomography imaging for reperfusion therapy in acute ischemic stroke
Noncontrast Computed Tomography for Intravenous Thrombolysis (NINDS and ECASS-3 for Intravenous Recombinant Tissue Plasminogen Activator, NOR-TEST and EXTEND-IA TNK for Tenecteplase, 0–4.5 Hours)
In 1995, the NINDS trial was the first prominent study to use anatomic CT scan-based selection of patients with AIS. This trial enrolled 624 patients with AIS with clearly defined time of symptom onset within 180 minutes, and all patients underwent an NCCT scan of the head before randomization. Patients with an AIS meeting these criteria were randomized to intravenous tissue plasminogen activator (alteplase) treatment or placebo, and the superior outcome in the alteplase group established the efficacy of IVT within 3 hours of symptoms onset. The purpose of requiring an NCCT scan in this trial was mainly to exclude the presence of intracranial hemorrhage, which was a contraindication to enrollment. An NCCT scan has a limited sensitivity to detect hyperacute ischemic changes in the first 6 hours post ictus, so the NINDS trial did not exclude patients based on signs of ischemic injury on the baseline NCCT scan.
In 2008, the ECASS-3 randomized, controlled trial was reported. The ECASS-3 asked whether IVT with alteplase was effective beyond 3 hours, and the results of this study extended the treatment window to 4.5 hours with stricter inclusion criteria (eg, an upper age limit 80 years). A total of 821 patients were randomized to intravenous alteplase versus placebo. Similar to the NINDS, an NCCT scan was required before administration of the thrombolytic to exclude presence of intracranial hemorrhage, but also to exclude a large established infarct that involved more than one-third of the middle cerebral artery (MCA) territory, which was thought to incur an increased risk of a large reperfusion hemorrhage. The ECASS-3 further cemented the importance of performing baseline brain imaging before the initiation of stroke treatment.
More recently, the Norwegian NOR-TEST tested the safety and efficacy of IVT with tenecteplase, which is a newer thrombolytic agent. In this study, 1107 patients with AIS were randomized to either tenecteplase or alteplase within 4.5 hours of symptoms onset. Similar to the ECASS-3, an NCCT scan was performed before randomization in all patients to exclude presence of intracranial hemorrhage and a large established infarct. The NOR-TEST study demonstrated noninferiority of tenecteplase compared with alteplase for IVT in patients with AIS.
The NINDS, ECASS-3, and NOR-TEST studies firmly established the importance of an NCCT scan for the evaluation of patients with AIS before IVT treatment. These studies also demonstrated how noninvasive brain imaging could lead to more precise selection of patients for treatment, and subsequent studies expanded the role of imaging before AIS treatment using newer EVT techniques.
Noncontrast Computed Tomography and Computed Tomography Angiography for Endovascular Treatment (MR CLEAN, THRACE 0–6 Hours)
Given the initial failure of EVT trials based on patient selection with an NCCT scan alone, subsequent trials used CTA to confirm presence of a large vessel occlusion (LVO) before EVT. MR CLEAN was a multicenter trial conducted in the Netherlands that randomized 500 patients with AIS with an National Institute of Health Stroke Scale (NIHSS) of at least 2 within 6 hours of stroke onset with confirmed LVO to either EVT plus standard medical care or standard medical care alone. Enrolled patients underwent an NCCT scan to rule out intracranial hemorrhage, and either CTA, MRA, or digital subtraction angiography to confirm presence of an anterior circulation LVO (MCA M1/2, anterior cerebral artery A1/2) before enrollment. EVT technique had evolved markedly after IMS III and SYNTHESIS expansion, and the vast majority of EVT cases (81.5%) in MR CLEAN were treated with mechanical thrombectomy using stent retrievers. , LVO recanalization at 24 hours was achieved in 75.4% of patients in the EVT group compared with 32.9% in the control group (odds ratio, 6.88). This recanalization translated into 32.6% of EVT patients having functional independence (modified Rankin Scale [mRS] of 0–2) at 90 days compared with 19.1% of control patients. MR CLEAN was the first randomized trial to demonstrate EVT efficacy for the treatment of AIS caused by an LVO.
THRACE was a randomized trial that compared AIS treatment by EVT plus alteplase versus alteplase alone. The study enrolled 414 patients with AIS with a confirmed LVO within 5 hours of symptom onset across 26 centers in France. Imaging selection was performed with an NCCT scan and either CTA or MRA to confirm the presence of LVO (MCA M1 or superior third of the basilar artery). EVT treatment was performed by mechanical thrombectomy, and intra-arterial tissue plasminogen activator was only allowed for cases with persistent distal occlusions. Of the patients in the EVT group, 53% achieved functional independence at 90 days (mRS of 0–2) compared with 42% in the alteplase alone group (odds ratio, 1.55).
MR CLEAN and THRACE provided important evidence that noninvasive vascular imaging by CTA or MRA before EVT is important for appropriate patient selection.
Noncontrast Computed Tomography Alberta Stroke Program Early CT Score/Computed Tomography Angiography for Endovascular Treatment (REVASCAT, 0–8 Hours)
The accurate delineation of ischemic injury severity on NCCT scan is challenging owing to the limitations of the technique. The Alberta Stroke Program Early CT Score (ASPECTS) was developed as a straightforward means to quantify the degree of ischemic changes on an NCCT scan. , ASPECTS is a 10-point scale in which 10 regions of a hemisphere affected by AIS are scored for the presence of significant hypodensity (ischemic injury). Patients with AIS with a higher ASPECTS have less injury at the time of imaging evaluation and were presumed to be more likely to benefit from EVT compared with patients with a lower ASPECTS.
The REVASCAT randomized, controlled trial compared AIS treatment by EVT plus medical therapy versus medical therapy alone in patients aged 80 years or older who presented within 8 hours of symptom onset. Enrolled patients had an ASPECTS of 6 or higher on an NCCT scan (or a diffusion-weighted imaging [DWI] ASPECTS of >5) and an LVO of the internal carotid artery or MCA (first or second segment) on CTA or MRA. These inclusion criteria were amended after 160 patients were enrolled, and patients 85 years old or younger and an ASPECTS of 8 or higher became eligible. Enrollment was halted after publication of the MR CLEAN trial, and an analysis of the 206 enrolled patients was performed. EVT resulted in higher rates of functional independence (mRS of 0–2) at 90 days compared with medical management alone (43.7% vs 28.2%; odds ratio, 2.1).
Noncontrast Computed Tomography ASPECTS/Computed Tomography Angiography Collaterals for Endovascular Treatment (ESCAPE, 0–12 Hours)
Ischemic injury quantification using ASPECTS in the REVASCAT trial was associated with superior functional outcomes compared with MR CLEAN, which suggests that more sophisticated neuroimaging selection criteria before EVT may lead to better outcomes. It has been suggested that the quality of collateral flow in patients with AIS might exceed the importance of occlusion duration, which challenges the long-standing dogma of “time is brain.” Consequently, patients with AIS with good collaterals as determined on CTA have been shown to benefit from EVT, whereas patients with poor collaterals may not benefit. This difference is likely due to rapid infarct growth within the first few hours after ictus in patients with poor collateral status.
The ESCAPE RCT compared AIS treatment by EVT plus medical therapy versus medical therapy alone in patients who presented within 12 hours of symptom onset. Enrolled patients had an ASPECTS of 6 or higher on an NCCT scan, an LVO of the internal carotid artery or MCA (first or second segment) on CTA, and evidence of moderate or good pial collaterals on CTA. Collateral status on CTA was preferentially assessed using a multiphase CTA technique that includes an arterial phase followed by mid venous and late venous phases. Collateral status was trichotomized into good (no delay or delay in one phase, but prominence and extent of peripheral vessels is the same as on the contralateral side), moderate (a 2-phase delay or a 1-phase delay and significantly reduced or absent peripheral vessels compared with contralateral side), or poor (few or no vessels visible within the ischemic vascular territory). In the absence of a multiphase CTA, moderate to good collaterals were defined as the filling of 50% or more of the MCA pial vessels on CTA. ESCAPE found that patients who underwent EVT had a higher rate of functional independence (mRS of 0–2) at 90 days compared with patients in the medical management arm of the study (53.0% vs 29.3%; odds ratio, 2.6).
Physiologic computed tomography imaging for reperfusion therapy in acute ischemic stroke
It is well-recognized that patients have widely different capacities to compensate for the lack of blood flow that occurs in AIS owing to LVO. Therefore, AIS treatment that is guided by an individual patient’s physiologic status may be more likely to result in superior outcomes after treatment. The ischemic brain may be considered in 2 compartments: an ischemic core (considered irreversibly injured) and the penumbra (salvageable if reperfusion of the brain is achieved). Advanced brain imaging that accurately identifies both the core and penumbra has been developed through imaging research over the past decades, and it allows treating physicians to identify patients with a target mismatch profile (small ischemic core and larger penumbra) who might benefit most from reperfusion therapy.
The ischemic core is best identified by diffusion-weighted MR imaging, but advances in CTP processing have allowed for largely accurate estimation of the core. The ischemic core is best delineated on cerebral blood flow or cerebral blood volume maps. By contrast, the penumbra is best identified as tissue with a time-to-maximum of the residue function (T max ) delay of 6 or more seconds or a mean transit time prolongation delay of 2.5 to 12.0 seconds. However, it is important to note that these thresholds vary with the postprocessing technique, which underscores the importance of validation at each individual scanner and stroke center. Cerebral perfusion imaging has been used in several studies to select patients for IVT or EVT, and these studies are summarized elsewhere in this article.
Computed Tomography Perfusion for Intravenous Thrombolysis (EXTEND, 4.5–9.0 Hours)
The EXTEND study sought to determine if patients with a target mismatch profile on cerebral perfusion imaging would benefit from IVT in extended time windows. EXTEND randomized 225 patients to either alteplase or placebo within 4.5 to 9.0 hours of symptoms onset or within 9 hours from the midpoint of sleep (if presenting with a wake-up stroke). Patients were enrolled after evaluation with cerebral perfusion imaging that identified a target mismatch profile (mismatch volume ≥10 mL and a mismatch ratio of ≥1.2) and a baseline ischemic core of less than 70 mL. The study found that patients who received alteplase were more likely to achieve an excellent clinical outcome (mRS of 0–1) compared with the placebo group (35.4% vs 29.5%), so there was a modest but statistically significant treatment effect.
Computed Tomography Angiography and Computed Tomography Perfusion for Endovascular Treatment in Early Window Patients (EXTEND-IA, SWIFT PRIME, 0–6 Hours)
The EXTEND-IA and SWIFT PRIME studies were randomized trials designed to determine if EVT and medical therapy was superior to medical therapy alone for the treatment of AIS owing to LVO in patients treated within 6 hours of symptom onset. Both studies were stopped early after the publication of MR CLEAN and found that EVT and medical therapy were superior to medical therapy alone.
The EXTEND-IA randomized, controlled trial enrolled 70 patients with AIS at the time the study was halted. Enrolled patients had (1) an anterior circulation LVO (intracranial internal carotid artery, MCA M1/2) as determined by CTA, (2) an ischemic core volume of 70 mL or less on DWI or CTP, and (3) the presence of a target mismatch profile on cerebral perfusion imaging (mismatch volume of ≥10 mL and a mismatch ratio of ≥1.2). EXTEND-IA found that patients in the EVT arm were more likely to achieve a good functional outcome (mRS of 0–2) at 90 days after treatment compared with the medical arm (71% vs 40%), and this study showed the largest absolute treatment effect among the early window EVT trials.
Similarly, SWIFT PRIME randomized patients with AIS with an anterior circulation LVO who had received alteplase to either undergo EVT within 6 hours of symptoms onset or to continue medical management. Enrolled patients had (1) an anterior circulation LVO (intracranial internal carotid artery or first segment of the MCA) as determined by CTA or MRA, (2) an ischemic core volume 50 mL or less on DWI or CTP, and (3) the presence of a target mismatch profile on cerebral perfusion imaging (mismatch volume of ≥15 mL and a mismatch ratio of ≥1.8). In addition, patients with a severe hypoperfusion lesion on cerebral perfusion imaging (T max of >10 seconds and a volume of >100 mL) were excluded. Midtrial, the imaging inclusion criteria were revised, and for the final 125 patients, centers could enroll patients based on CT or DWI ASPECTS of greater than or equal to 6, even though the use of perfusion imaging was encouraged. SWIFT PRIME demonstrated superiority of the EVT arm (mRS of 0–2 at 90 days of 60% vs 35% in the medical arm) after being stopped early.
Computed Tomography Angiography and Computed Tomography Perfusion for Endovascular Treatment in Late Window Patients (DAWN and DEFUSE-3, 6–16 or 24 Hours)
If the concept of infarct core and penumbra is scientifically sound, one would expect that patients with AIS with significant penumbra would benefit from recanalization even in late time windows. Importantly, the presence of significant penumbra has been confirmed at 24 hours after symptoms onset and event beyond 24 hours. The late time window EVT trials, DAWN and DEFUSE-3, asked if patients who present between 6 to 16 hours (DEFUSE-3) or 6 to 24 hours (DAWN) after symptom onset benefit from EVT.
The DAWN trial enrolled patients within 0 to 24 hours of symptom onset with an anterior circulation LVO (internal carotid artery or first segment of the MCA) on CTA or MRA who had a mismatch between the volume of the ischemic core and the severity of their symptoms. DAWN randomized 206 patients to either EVT plus medical management or medical management alone. The ischemic core volume was determined by either DWI or CTP, and the volume of core allowed for enrollment varied by patient age. In patients 80 years or older, an NIHSS of 10 or higher and a core infarct volume of less than 21 mL were required. In patients less than 80 years of age, an NIHSS of 10 or higher and a core infarct volume less than 31 mL, or an NIHSS of 20 or higher and a core infarct volume of 31 to 51 mL were required. The trial was halted because an interim analysis revealed the superiority of the EVT arm. Functional independence (mRS of 0–2) at 90 days was 49% in the EVT group compared with 13% in the medical care alone group. Subgroup analyses revealed a robust treatment effect at 6 to 12 hours and 12 to 24 hours after symptoms onset.
The DEFUSE-3 trial enrolled patients with AIS within 0 to 16 hours of symptom onset with an anterior circulation LVO (internal carotid artery or first segment of the MCA) on CTA or MRA, an ischemic core volume of less than 70 mL, and the presence of a target mismatch profile on cerebral perfusion imaging (mismatch volume of ≥15 mL and mismatch ratio of ≥1.8). DEFUSE-3 randomized 182 patients to either EVT plus medical therapy or medical therapy alone. The trial was halted when the results of the DAWN Trial were presented, and an interim analysis stopped the study for efficacy in the EVT treatment group. Of patients in the EVT group, 45% were functionally independent (mRS of 0–2) at 90 days compared with 17% in the medical group.
DAWN and DEFUSE-3 were landmark trials that demonstrated the effectiveness of physiologic imaging to guide EVT treatment decisions in late time windows, and these studies opened up EVT treatment eligibility to a much larger number of patients.
Gaps in knowledge and ongoing acute ischemic stroke trials
Despite the explosion of AIS trial data that have demonstrated the efficacy of both IVT and EVT after appropriate imaging selection, there are many unanswered questions. Whether patients with mild symptoms (NIHSS of ≤6), large ischemic cores (≥70 mL), old age (≥90 years), or posterior circulation LVOs (vertebral or basilar artery) benefit from EVT or whether cerebral perfusion imaging may identify other populations who might benefit from IVT remains to be determined. There are several ongoing trials that hope to answer some of these questions, but other questions will require further investigation. We briefly summarize several ongoing AIS trials that have not yet been reported ( Fig. 1 ).

Intravenous Thrombolysis Trials
TEMPO-2 is a randomized trial that will test if IVT with tenecteplase is an effective treatment for minor strokes (NIHSS of <6) in patients presenting within 12 hours of symptom onset. A head CT scan that demonstrates ASPECTS of greater than 7 as well as a CTA (or CTP) that confirms an intracranial occlusion (MCA, anterior cerebral artery, posterior cerebral artery, vertebrobasilar) are the imaging inclusion criteria.
TASTE aims to further validate the mismatch hypothesis for IVT by enrolling patients who are eligible for IV tissue plasminogen activator by standard criteria (<4.5 hours from symptoms onset) who also undergo CTP that demonstrates a core infarct of less than 70 mL, a mismatch ratio of greater than 1.8, and a penumbra volume of greater than 15 mL.
TIMELESS will compare tenecteplase with placebo for the treatment of anterior circulation patients with AIS who present between 4.5 and 24 hours after symptoms onset. Imaging enrollment criteria include a CTA (or MRA) that demonstrates an occlusion of the internal carotid artery, MCA (M1 or M2 segment), and CTP (or DWI and MRP) that shows an ischemic core less than 70 mL, a mismatch ratio of 1.8 or greater, and a mismatch volume 15 mL or greater.
Endovascular Treatment in Patients with Acute Ischemic Stroke with a Large Infarct Core
A post hoc analysis of THRACE suggested that good outcomes are achievable in some patients with ischemic cores of greater than 70 mL. Similarly, SELECT, which was a large prospective EVT study, provided evidence for an EVT treatment effect in patients with ischemic cores of greater than 50 mL or CT ASPECTS of less than 6 on baseline imaging. These data are consistent with a meta-analysis of all major EVT trials that demonstrated that ischemic core size is independently associated with clinical outcome, but that it does not modify the treatment benefit of EVT. The same group also has demonstrated that patients with AIS with a variety of baseline imaging characteristics, including infarct volumes of greater than one-third of the MCA territory and ASPECTS 3 to 5 (but not ASPECTS 0–2), benefit from EVT. These data challenge the widely used infarct core thresholds of less than 50 mL or less than 70 mL. , It is likely that the relationship between outcome, EVT efficacy, and a large core is more complex. Consistent with this idea, a post hoc analysis of 205 DAWN patients demonstrated a bidirectional treatment effect in patients with AIS with CT ASPECTS of 0 to 6, where EVT was associated with both a greater chance of acceptable (mRS of 0–3) outcomes (50 vs 25%), but also a higher chance of poor outcome (mRS of 5 or 6; 40% vs 25%). Although EVT may be beneficial in patients with AIS with large ischemic cores, additional study is necessary.
Currently, there are 4 ongoing EVT trials that are investigating the benefit of EVT in patients with AIS with large ischemic cores. First, TENSION is enrolling patients with CT or MR ASPECTS of 3 to 5 last seen well within 12 hours of symptoms onset. Second, SELECT-2 is enrolling patients with CT ASPECTS of 3 to 5 or a CTP/DWI core of more than 50 mL. The inclusion criteria for SELECT-2 include anterior circulation LVO in patients with AIS who present within 0 to 24 hours from symptom onset. Third, LASTE is enrolling anterior circulation LVO patients with AIS within 6.5 hours of symptoms onset with CT or MR ASPECTS 0 to 5 (if <80 years) or CT or MR ASPECTS of 3 to 5 (if >80 years). Last, TESLA is enrolling anterior circulation LVO patients with an NIHSS of at least 7 within 24 hours of last seen well and a CT ASPECTS of 2 to 5. The results of these trials will help to determine whether treatment of patients with large ischemic cores results in improved outcomes.
Endovascular Treatment in Mild Strokes
Some patients with AIS with an LVO may present with relative mild symptoms (NIHSS of 0–6), and whether these patients benefit from EVT is unclear. It is estimated that up to one-third of these patients will deteriorate and ultimately have a poor outcome if left untreated. , Several studies have suggested that these patients benefit from EVT, , , whereas others have not. A meta-analysis by the HERMES group also did not show a clear benefit of EVT in patients with AIS with an NIHSS of 0 to 10. High-quality data are needed to guide EVT treatment decisions in patients with AIS with an LVO and mild symptoms.
Endovascular Therapy for Low NIHSS Ischemic Strokes (ENDOLOW) is a randomized trial that will compare EVT with medical management in patients with AIS with an LVO who present with mild symptoms (NIHSS of 0–5) within 8 hours of symptom onset. The imaging inclusion criteria include CT ASPECTS of 6 or higher or an ischemic core (determined by CTP or DWI) of less than 70 mL.
Suggested imaging algorithms for selection of patients with acute ischemic stroke based on randomized, controlled trial data
We have reviewed a number of AIS trials and their varied imaging inclusion criteria. Key IVT trials and their inclusion/exclusion criteria are also summarized in Table 1 . For key EVT trials and inclusion/exclusion criteria, please refer to Table 2 .