Fig. 22.1
(a) Optical Tracking System. (b) Optical Tracking System placed over the head of the bed for optimal “line of sight”
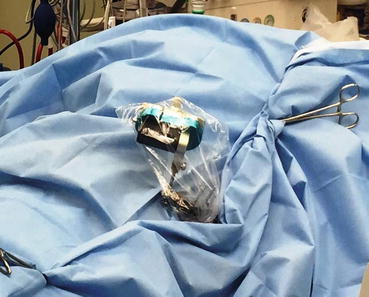
Fig. 22.2
Tracker attached to the pelvis with three pins with the patient in the prone position; the tracker is oriented to see the camera. It is important to appreciate line of sight and avoid drapes, tubing, and electrocautery wiring blocking the view of the camera
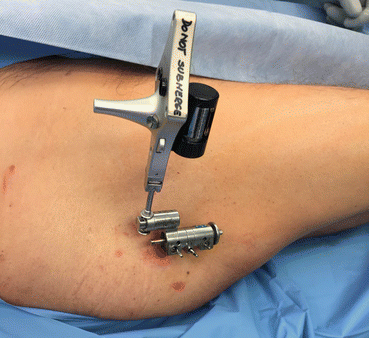
Fig. 22.3
Alternate tracker sometimes used if unable to register with the standard patient tracker
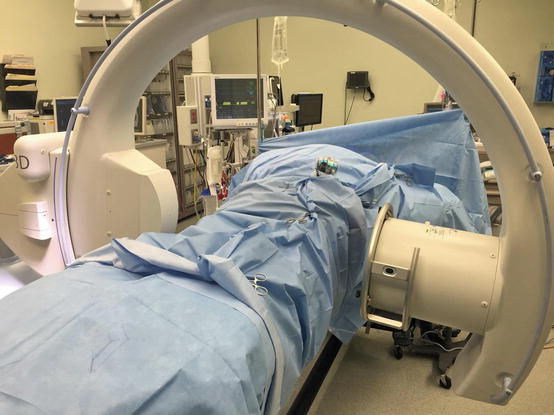
Fig. 22.4
Arcadis Orbic 3D fluoroscopic system
22.5 Computer Navigation Process
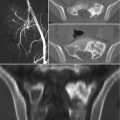
Preoperative Process: The use of computer navigation starts with obtaining 3D imaging of the tumor in question. Navigation of the pelvis and sacrum cannot be performed with imageless referencing, and thus all this navigation is based on CT, MRI, and CT-MRI fusion imaging. This is because “imageless navigation” uses the pelvic plane and bony landmarks for reference and soft tissue on the pelvis renders this method fairly inaccurate [48–50]. In order to use computer navigation, the entire bone must be imaged. While theoretically one could just image the sacrum, it is very hard to identify specific bony landmarks on the sacrum intraoperatively, and thus the entire pelvis is often imaged. Bone tumor resection requires several important steps in order to make the osteotomy almost effortless. Fluoro-CT matching and 3D fluoroscopy may allow for more accurate resections because soft tissue on the sacrum and pelvis can distort the ability to use surface matching and paired-point matching [24, 42]. Newer navigation systems exist with the use of cone-beam computed tomography or O-arm that allows 3D image quality with submillimeter spatial resolution with less radiation dose that can be used repeatedly in the operating room [27, 51]. The most important study to obtain is a preoperative CT with or without fiducial markers.
The purpose of navigation is to identify the bony margins for resection, and thus a CT scan is used because it is the best modality for cortical bone. MRI and CT have a high sensitivity and specificity for bony tumor infiltration but margins are difficult to identify intraoperatively and intralesional resection is very much possible in the sacrum [29]. Currently, surgeons not using navigation rely on two-dimensional imaging consisting of a CT and MRI obtained preoperatively and then analyze and reconstruct the images into a 3D model within their mind during the intraoperative resection leading to significant inaccuracies in tumor resection [31, 39, 40]. While CT is often better at visualizing cortical bone status, MRI (Fig. 22.5) is the best method for defining marrow involvement for bone tumors and soft tissue sarcomas when planning for resection margins [52]. A surgeon can measure on MRI the marrow infiltration and then recreate this measurement on the CT used in computer navigation as long as a similar slice thickness is used. In general, when we perform surgery in the pelvis and on the sacrum using computer navigation, we typically only use the CT imaging although some will use a CT-MRI fusion [1, 2, 11, 52]. We can see some of the soft tissue on CT although not as well as on an MRI. While we have not taken advantage of CT-MRI fusion, this modality is probably the best way to look at soft tissue when using computer navigation. CT-MRI fusion allows for determining the extent of tumor resection planes based on the bony involvement seen on the navigated software [25, 26]. CT-MRI fusion use has been described and was felt to be beneficial to the surgical procedure [24]. Most fiducial markers in the past were K-wires or titanium screws not allowing for the MRI to be used in preoperative imaging for navigation. Some studies have described using resorbable 1.5 mm pins placed beyond the tumor resection so that CT-MRI fusion and MRI images alone can be used for patient registration [52]. While MRI alone can be used as an intraoperative guide, its use in registration for navigation requires the use of paired-point registration but obtaining a registration error of <1 mm is hard without using fiducial markers [2] and metal markers cannot be used due to artifact associated with MRI. An important point several authors make is that when using fiducial markers the slice cut must be less than the slice thickness of either the preoperative MRI or CT scan; otherwise, the possibility exists that one may miss the fiducial marker on the scan [52]. The image fusion process is not without its own contribution to registration errors because merging is still done visually by the surgeon leading to potential error even with the best processes producing errors of 6 mm or more [52, 53].
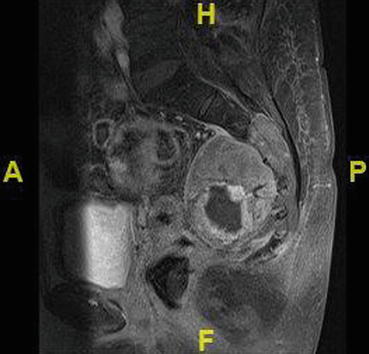

Fig. 22.5
Contrast MRI of the sacrum showing bone infiltration not easily appreciated on an X-ray
Next comes planning on part of the surgeon through the aid of MRI for soft tissue component and bony peritumoral edema and as well a CT for bone component to determine the resection level. A specific CT consisting of a protocol for navigational software must be obtained in order to be imported into the navigation or computer-assisted software. At a minimum, a CT scan of the entire affected bone must be obtained that has 0.5–2 mm cuts, continuous, and with no overlap [20, 28, 31, 39, 40, 42]. Virtual planning comes at the time when a CT scan is imported into the navigational software and the surgeon elects planes to determine the starting point and vector of the intended plane for future osteotomy or resection plane (Fig. 22.6). This all occurs prior to the patient coming to surgery and is determined by the interpretation of the preoperative imaging at a minimum consisting of a CT, but can include an MRI.
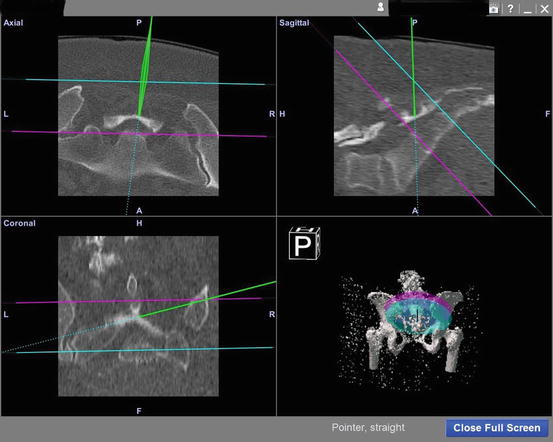

Fig. 22.6
Navigation screen showing tip of navigated instrument in green; the other planes represent the sacral joints in several different planes. This figure shows the navigated pointer and its use to identify the beginning of the resection level. The other planes were created during preoperative planning in order to avoid resection above or below these levels
Intraoperative Process: The intraoperative process consists of tracker placement, registration, and then resection. Intraoperative registration can occur at one of two time points, both of which are after the patient has been put under general anesthesia. Either at the beginning prior to major surgical incision or at the time prior to resection after all is exposed. This is determined by the technology available or the surgeon’s preference on whether they think registration through “paired matching” based on surface landmarks will be accurate enough or whether a reconstruction by fluoroscopic “spin” with a C-arm would be more accurate.
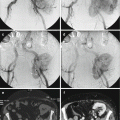
When performing surgery with computer navigation, it is important to understand tracker placement. Optical trackers can have issues with line of sight (Figs. 22.1 and 22.2), and it is important to think about surgeon’s and patient’s positioning and use of navigated tools [54]. Currently, at our institution, we have used an optical tracker with infrared sensors but we were forced to be cognizant about tracker placement as well as tracker and tool direction. Often the longest pins are used and placed into a stable part of the pelvis away from the surgical site, usually on the contralateral side of the pelvis [55]. The tracker can be placed into the non-resected sacral or pelvic bone or can be placed into the resected bone if one chooses. However, if one needs to reconstruct or re-resect based on frozen margins, placing the pins in the resected section will not work. Although two pins can be used for tracker fixation, it is best to use three pins to better stabilize the tracker [56]. If the tracker pins become loose, it can affect the registration accuracy and the registration process will have to be performed again [20]. Also one must understand that the further the tracker is placed away from the resection plane or the bony sacrum or pelvis the more room there is for induced additional error in the system [45, 57]. The tracker should be placed far enough away from the surgical site so minimal disturbance happens to the system during surgery, i.e., leaning on the tracker with a retractor. We have placed pins and both removed and left the tracker attached during surgery prior to resection but after registration. After registration and prior to resection, we either place the tracker back on and or touch point on the pelvis or sacrum to confirm on the imaging that where we place the pointer is where we are on the computer navigation screen. Anecdotally, no difference in accuracy between the two methods has been seen although this hasn’t been formally tested. Decreased accuracy will develop when the tracker or pins have been leaned on or when the pins become loose. When this happens, rescue points or respinning can be used to re-establish accuracy of the tracker. Rescue points are chosen on the computer navigation system prior to surgery. A minimum of four rescue points are chosen that are accessible, identifiable, and reproducible and are often prominent bone landmarks, i.e. ASIS, AIIS, PSIS, and pubic tubercle and pubic symphysis and one can use fiducial markers that are bioabsorbable.
One of the main logistical and timing issues in sacral surgery is often the need to flip the patient if using anterior and posterior approaches. This becomes a major issue with computer navigation as the most accurate way to match the CT with the patient in surgery is through a “spin” (Fig. 22.4). The benefit to navigation is that sometimes allows surgery to be performed through one approach. We often perform only one 3D fluoroscopic spin at the beginning of surgery with only tracker site incision opened up and the patient partially draped. The patient was covered with sterile sheets and the tracker with a sterile clear bag (Fig. 22.2). After the spin is completed with the Arcadis C-arm, we finish draping the patient which includes Ioband and thus only the pin sites were exposed to infection risk. This theoretically minimizes our risk of infection at the actual surgical site. This is probably less of a concern in sacral surgery unless one is performing lumbar-pelvis fixation. Each time the patient is flipped and repositioned the tracker must be replaced and new spin can be performed but it most likely places the patient at a theoretically increased infection risk although placing the patient on fresh surgical incision probably is a bigger risk. In this situation or having to flip back and forth more than once, screws can be placed in either planned resected bone or in other areas of the pelvis that can then be marked on the computer navigation software that can be used as well-defined rescue points, at least four of these have to be chosen. The benefit to fiducial markers is that another spin need not be performed but rather the fiducial markers can be marked as rescue points in order to alleviate the need for another spin.
Registration: All CT-based navigation systems require a registration process prior to navigated surgical resection [58]. Registration is the most significant and error prone step in navigation [59]. Originally, there were two registration algorithms developed for CT-based navigation paired-point matching and surface matching [58, 60] and now there is noninvasive registration of 3D data sets with the use of a navigated 2D fluoroscopy via contour matching first described in the spine [60]. This occurs after a tracker has been placed in a position that will be stable throughout surgery [20, 22]. This can be done in several different ways and is mostly based on the type of computer navigation software present at each institution with most systems being commercially available. Since 2008, we have used a commercially available system (OrthoMap, 3D, Styrker Orthopaedics, Mahwah, NJ) at our institution. This software package allows the surgeon to input data from both a CT and MRI obtained preoperatively several days before surgery. In the surgical suite, we take advantage of the Arcadis C-arm (Siemens), a 360 degree fluoroscopic image intensifier that allows the clinician to use a C-arm to perform a spin to obtain multiple multi-planar fluoroscopic images focused on the pubic symphysis (Fig. 22.4). This 3D fluoroscope turns around a surgical bed in a 190° arc acquiring up to 100 images of the iso-center with a dimension of 12.5 cm3. With these axial cuts, 2D and 3D reformations can be transferred into the navigation system (Fig. 22.7) [61]. In order to use 3D fluoroscopy for registration, a radiolucent bed is required to allow the spin to occur. The limitation of this process is the small fluoroscope field (12.5 cm) and the need to spin around a radiolucent bed requiring that the surgeon use a centered anatomic structure of the patient in order to match the registration, i.e., the pubis. The C-arm obtains several hundred images that form a pseudo 3D CT image that is then matched with the pubic symphysis (Fig. 22.7) on the preoperative CT loaded into the navigation software to create a virtual model to guide the surgeon. This process can take up to 20–30 min to perform and requires sterilely covering the draped sterile field. The other intraoperative data we collect are surgeon-defined landmarks to confirm accuracy of the “spin” as well as defining optimal resection levels and implant positioning if needed. Image-to-image registration using 3D fluoroscopy [42] offers the benefit of not requiring fiducial markers along with a pre-resection surgery and can have improved registration accuracy over paired-point matching using bony landmarks. Although target registration error is the smallest when using the ilium (centered 3 cm above the acetabulum), one often has to use the pubis symphysis due the inability of a C-arm to be centered over ilium at the supra-acetabular location and complete an unimpeded revolution around the bed depending on the type of 2D fluoroscopic arm [42].
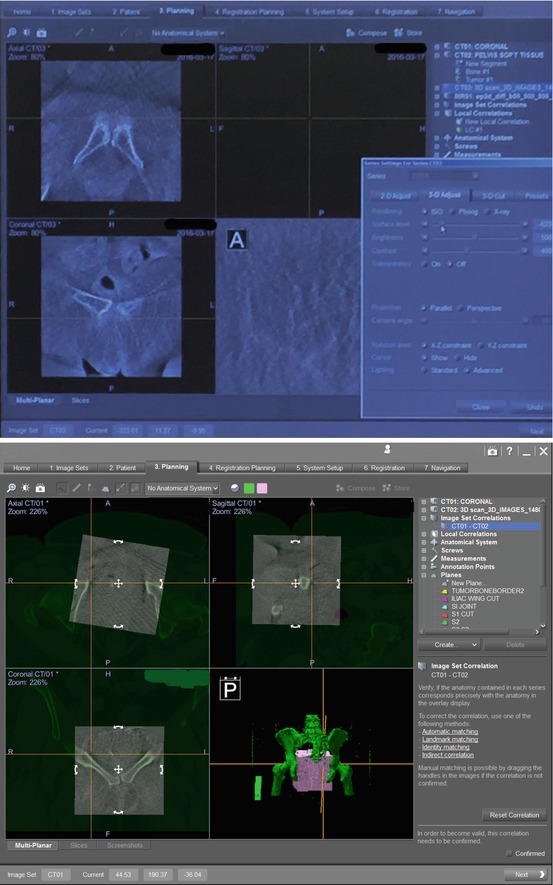
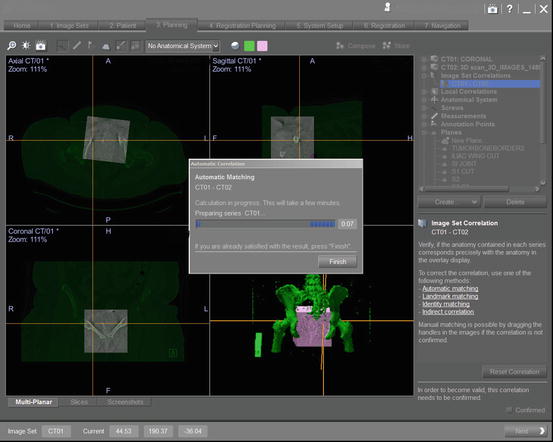


Fig. 22.7
This is a screenshot showing the navigational computer screen after uploading the imaging obtained from the Arcadis fluoroscopy machine. This imaging created by the navigational computer is used to merge the pubic symphysis seen on the preoperative CT scan and intraoperative “spin”
Other methods exist and include “paired-point matching and requires a minimum of 4 points or paired points” that are chosen on the CT image and then identified in the patient [22, 25, 26, 28]. This requires the surgeon to accurately identify both on the CT image and on the patient the corresponding points. Oftentimes, the pubic symphysis, ASIS, AIIS, pubic tubercle, and PSIS are used for the patient to image matching [55]. Many will confirm the registration process by palpating pubis and ilium [31, 55]. However, there are specific bony landmarks often that cannot be identified or palpated due to patient positioning.
Accuracy of the registration process can be improved by surface matching [23]. Surface matching was developed to avoid a second surgery and improve efficiency [58, 62]. The surgeon used the navigational probe to select in continuous succession a minimum of 50 points on the patient’s exposed bone surface of the pelvis or sacrum [22, 28]. Cartilage, ligament, and soft tissue cannot be used for surface mapping due to the fact that they will not show up very well on a CT scan. CT images can be used for surface registration due to the nature of the bony cortex [25, 26]. MRI, on the other hand, can’t be used for surface registration because the navigation system has a difficult time recognizing the cortical surface on the MRI [1, 52].
The registration error can be calculated by the navigation software and gives an indication of the mismatch between the true anatomy of the patient and the virtual image created by the preoperative CT scan. The goal is to obtain a registration error of <1 mm but some will accept below 2 mm [11, 22, 25, 26, 28, 63, 64]. Not too frequently a registration error of greater than 2 mm will be obtained. When this occurs, it often happens due to soft tissue being in the way of a bony landmark or that the paired points were not correctly matched from the preselected area on the preoperative CT scan. Having an obese patient can make it hard to identify the correct points. The surgeon will then have to repick points after confirming in the navigation system he has picked his correct landmarks on the CT scan. After this, the process is repeated again to correctly obtain point on the pelvis. Rarely does one have to abandon navigation. Because the “paired point” process can lead to user error, our institution chooses to use a “spin” method (discussed above) performed by recreating a bony landmark such as the pubic symphysis through the use of a C-arm taking several hundred images of the pubic symphysis that allow a virtual 3D image to be created that can be matched with the CT imaging. Tracker insertion and registration can take between 15 and 47 min but can often decrease from an average of 30–20 min after the surgeon has performed more navigated surgeries [28]. Time is then saved during surgery by not having to bring in C-arm or X-ray for multiple orthogonal images.
Fiducial markers are another way of enabling accuracy of registration; an implant such as a titanium implant, either a Kirschner wire [1] or screw, is placed into the patient’s pelvis under anesthesia prior to obtaining a CT scan of the pelvis for navigation. These serve as fiducials for patient registration at the time of surgical resection [20]. Due to the possibility of needing to flip the patient, which can cause issue with registration and thus accuracy, surgeons in the past have placed fiducial markers consisting of K-wires (Titanium 1.8 mm) [20] into the pelvis prior to obtaining CT imaging for navigation. The points are chosen based on ease of access during surgery such as the iliac crests and posterior iliac spines [2, 20]. No more than four fiducial markers are needed for an accuracy of 1.5 mm [65]. Fiducials allow for better paired- point registration than would be obtained with identifying bony landmarks. This can then be augmented with surface fit registration. Planned registration landmarks should not be prominent osseous features that may be included in the resection [52].
To improve accuracy with paired-point matching, small pins or fiducial markers can be attached to the anatomical surface of interest but an additional surgery to place these markers must occur prior to the preoperative CT [42, 58]. Fiducial markers can make things easier or if the patient is needed to be flipped from supine to prone, not all the time can the surgery be performed only through a posterior approach [54, 66]. While not routinely done, there are reported series of performing a surgery prior to resection to place fiducial markers on or within the pelvis prior to obtaining a CT scan for navigational planning. It is important to understand that a surgery needs to be performed prior to obtaining the preoperative CT scan loaded into navigation. These offer the ability to identify a finer paired-matched point when performing registration. However, this places the patient as risk for infection although it is really mostly percutaneous. It also puts the patient through another anesthesia.
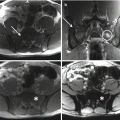
Performing the resection: After reconfirming ones observed accuracy from the registration process, bone cuts can be made several ways with navigation. Instrumentation exists that allows a tracker to be placed onto a designated tool, and custom tracker connectors can allow about any type of tool to be navigated as long as it can be calibrated. Several different tools can be used for navigation and can be navigated and include diathermy device [22, 28], osteotomes [21], chisels [10, 19, 20], drills [25, 26, 67], burrs [2, 11], screw driver [21], and oscillating saws (Fig. 22.8) [22, 24, 31, 68]. Two of the most common tools used include a navigated saw and navigated osteotome. The navigational saw involves placing a tracker on a conventional battery-driven saw that has a specific blade that is much narrower than a saw blade used to perform a knee arthroplasty. The drawback of this saw blade is there is some significant flexibility in the system. Instability due to vibrations and flexibility produced by the oscillating saw can potentially induce error in the planned resection [51]. The operator can try to push the saw blade and cause flexion that can cause an inaccurate reading of the saw blade in space to the effect of several millimeters [39, 40, 68]. The saw also has to be started away from the cortex. The key is to let the saw blade do the work. The other limitation of the saw is the excursion of the blade and often cannot be used if the blade cannot oscillate or if oscillation will injure tissue or disrupt tumor [22]. There are significant improvements for angle of cut and location of the cut plane (2.8 mm) when using a navigated saw compared to the freehand process (5.7 mm) [69]. The other navigational tool at hand is the osteotome that allows the user to have non-flexible tool that gives reliable depth and trajectory feedback via the computer navigational screen to the surgeon (Fig. 22.9). The drawback of the osteotome is that using it can cause unwanted fractures in bone. Both the saw and the osteotome are ideal for uniplanar cuts but when a multi-planar three-dimensional cut is needed another method is used. The surgeon can use the navigation pointer/gun to mark “wayward” points along the bone for the planned resection performed with either a drill, burr, or osteotome. These points after being identified with the navigated pointer [1] can be marked with cautery or a sterile marker which has been described [4, 25, 26, 39, 40]. At this point, a drill or a burr can be used to create several holes along the planned cut. A burr has benefit over a drill in that it can be used to thin cortex on the far side before dropping into tumor or a critical structure that may exist on the opposite side (Fig. 22.10). The cut can then be completed with either an osteotome or burr at the discretion of the surgeon. Once the integrity of what bone in question is compromised, i.e., resected then the accuracy of the navigation is no longer valid. This occurs once the pelvis is disrupted in one area; there is too much flexibility in the system to assure that the second osteotomy is in the intended place [28]. Disruption of the ring can potentially disturb the accuracy of the spatial relationship and the registration of the patient [2]. One thing to be aware of is that the display of the instrument on the monitor and real localization may differ with respect to what is seen in the operative field [38].
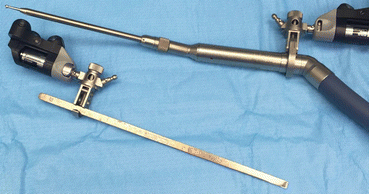
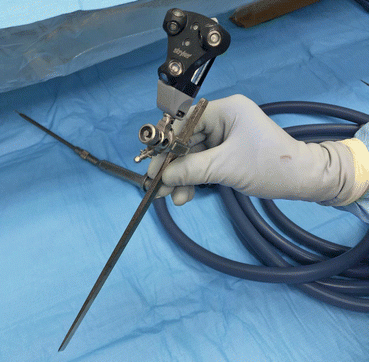
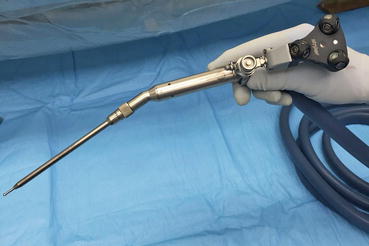

Fig. 22.8
Comparison of a navigated osteotome or chisel to a navigated burr. Make note the burr is not straight; the burr tip is referenced to the instrument tracker relative to space

Fig. 22.9
Navigated osteotome 1/4 in. (1/4 to 1 in. can be navigated)

Fig. 22.10
Navigated burr used for resection in multiple planes; generally, the smallest size burr is used to avoid compromising margins
22.6 Benefits of Navigation
Computer navigation is a real-time intraoperative virtual imaging system that allows the surgeon to identify location and planned resection in musculoskeletal oncology (Fig. 22.11). The system can allow the user to make uniplanar resections with confidence as it allows one to know the exit point of the cutting device (Fig. 22.12). Previously, these cuts would have been made visually or with the aid of two-dimensional radiograph or fluoroscopy. Those two modalities often do not allow the surgeon to know how far or deep one has a cut. In the past and still today with sacral surgery, a surgeon may attempt to place screws in the sacrum on the opposite side to be able to identify on a plain radiograph or fluoroscopic image in order to make a relatively safe blind cut. The issue with this cut is that x-ray and fluoroscopic image doesn’t allow the surgeon to identify soft tissue component of the tumor on the other side. With the use of computer navigation the surgeon can now save nerves and blood vessels and create multi-planar resections that allow for preservation of bone and thus improved the ability to implant attachment if needed. Musculoskeletal tumors are not one dimensional, they don’t follow a single plane border, instead they are often lobulated three-dimensional masses thus making the resection harder. The other benefit of navigation is the ability to navigate multiple tools. A surgeon can now know the depth and direction of their tool in three-dimensional space. Computer navigation doesn’t only have to be used for resection and reconstruction, stealth navigation with the O-arm and single K-wire for sacral and pelvic lesions that represent benign tumors and hematologic tumors were used for tumor ablation and kyphoplasty in benign and malignant non-primary bone tumors [27]. Another theoretical benefit of navigation compared is decreased fluoroscopic exposure times to the surgeon [61, 70].
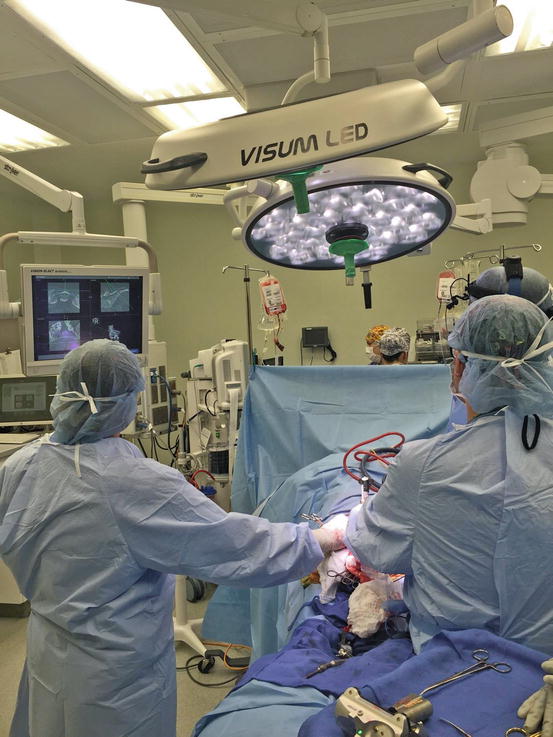
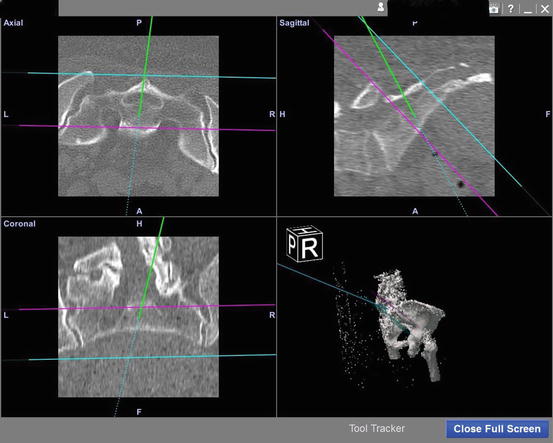

Fig. 22.11
Two surgeons watching the computer navigation screen while using a navigated burr on and in the sacrum for a chordoma resection

Fig. 22.12
The green tip represents the tip of the navigated burr and shows how deep it has gone
Accuracy Benefits: Computer navigation surgery allows the surgeon several benefits with regard to surgery and margin status. In surgeries without navigation, surgeons often have to plan 2 cm margins in order to be assured that there is no tumor violation [66]. It is difficult to achieve negative margins in sacral surgery [66] and tumors involved with the sacrum have a higher prevalence of positive margins [71] leading to higher recurrence rates and poorer outcomes. Clinical studies have shown that navigated tools have assisted in attaining negative margins in pelvic and sacral tumors [2, 10, 24–26, 31, 72]. Freehand-navigated saw improves cutting accuracy [51, 73]. Computer-navigated surgery can increase precision of the osteotomies for tumor resection [1, 2, 10, 20, 25, 26]. Given the accuracy of navigated tools, bone loss related to the saw blade thickness can be accounted for and then adjusted for on computer navigation planning by shifting the planned resection planes by 1.5 mm [31]. In fact, most resection planes can be adjusted preoperatively based on known resection width tools such as the osteotome (0.6 mm) and oscillating saw blade (1.25 mm) which produced a loss of bone width of 2 mm due to oscillation [51].
The use of computer navigation allows for accurate identification of the local anatomy in a distorted environment and can define the extent of the tumor and resection margins [28, 39, 40]. Oftentimes, it is hard to identify the location and the extent of tumor infiltration intraoperatively [39, 40]. A surgeon without computer navigation can’t see the infiltration of the tumor within the bone marrow intraoperatively and instead the surgeon must identify on MRI and CT the tumor infiltration related to bony landmarks and translate that into landmarks on the patient’s pelvis or sacrum during surgery; this relationship between what is seen on preoperative imaging and what is identified in the patient can lead to errors that translate into positive margins in the non-computer-assisted surgery. Computer navigation allows a surgeon to identify the extent of the tumor infiltration on the virtual imaging that is shown on the computer navigation monitor and know that their resection will not go through tumor. The main benefit of improved accuracy through this modality is the ability to get closer to the tumor without compromising margins. The goal of navigation is to reduce the rate of intralesional resection, i.e., positive margins. One can study looking at navigation in the pelvis and sacrum reduced the intralesional rate to 8.7% (n = 2) with clear bone margins in all cases (n = 31) with a 13% local recurrence rate compared to the traditional method where intralesional rate and local recurrence rate were found to be 29% and 27%, respectively [22]. Only now are oncologic surgeons beginning to see the benefit of getting closer to the tumor without compromising the margins. Surgeons using computer navigation believe that it allows more complex resections and reconstructions than are possible with conventional surgery including preservation of sacral nerve root controlling bladder and bowel (42% of time), resect unresectable tumors (13%), and avoidance of hindquarter amputation (10%) [22].
In studies, there is a 52% probability of achieving a 1 cm margin in a triplane-simulated tumor model of the pelvis when performed by an experienced surgeon without computer navigation [30]. One experimental study showed that the cut planes with respect to the planned planes in the pelvis were significantly improved by using a navigated saw, averaging 2.8 mm compared to 11.2 mm for the freehand saw (p < 0.001) and there were no intralesional tumor resections compared to 22% (N = 5) intralesional procedures in the freehand group (N = 23) [68]. What was also found within this experimental study is that the maximum difference achieved between the cut and the desired safe margin of 10 mm was 6.5 mm for the navigated cut compared to the 13 mm cut [68]. In another experimental study in the setting of using intraoperative CT, the navigated sawbones cuts were 1.4 ± 1 mm entry cut and 1.9 ± 1.2 mm exit cut from the planned resection compared to non-navigated 2.8 ± 4.9 mm entry cut and 3.5 ± 4.6 mm exit cut in a pelvic bone model showing a significant (p ≤ 0.01) difference in the two methods and the navigated cadaver study produced similar values for the navigated entry cut location of 1.5 ± 0.9 mm and navigated exit cut location of 2.1 ± 1.5 mm form the planned cuts using [51]. This showed that navigation used in pelvic tumor resection allowed an osteotomy within 5 mm of the planned resection [51]. The overall benefit to navigated surgery is the reproducibility of the surgical resection. In a study of 28 patients with 61 osteotomies, the quantitative difference between the planned osteotomies and performed osteotomies was 2.52 ± 2.32 mm for all patients and 2.82 ± 2.01 for the sacrum and pelvis [39, 40]. While not often used in the setting of sacral tumor allograft, reconstructions can benefit from the use of computer navigation. Inaccuracy with respect to measured dimensions exists in the selection of massive bone allograft of the pelvis using template comparison method [74]. Due to the reproducibility of the precision, some surgeons use navigation to resect the tumor and then use the navigation software to create an allograft piece of pelvis or sacrum to fill the defect created from the resection in order to reconstruct the pelvis. Navigation has been shown that it can also be used to cut a custom fitting allograft if reconstruction is to be performed [31]. One of the original purposes of navigation that is not often used in sacral resection is reconstruction of the appropriate hip center, leg length, and hip joint version. It allows a way to properly orient reconstruction components when no bony landmarks exist.
22.7 Limitations of Navigation
Limitations of Accuracy: Navigation is not a perfect system and the surgeon should understand what affects its accuracy in surgery [13]. Accuracy can be limited in several ways: the imaging system itself can limit accuracy, operator error of the tools, and registration of the tools and registration of the patient can limit accuracy. Inaccurate osteotomies or lack of precision for a cut can be manually caused by the surgeon due to saw blade bending when cutting bone [69]. Be aware that the instrument location seen on the display may not match the real localization on the patient [38]. Some studies have reported that the true accuracy in most studies only represent the accuracy of the intraoperative registration process as recorded by the navigation machine [24] and have even suggested that pathology margins are the best way to measure accuracy. Given that most inaccuracies occur due to the registration process which is operator dependent, a surgeon should understand the affect that they impart into the system. Getting good information out of computer navigation is dependent on putting good data into the system. Patients with significant BMI [28] can limit exposure and make it difficult to accurately identify bony landmarks for paired-point matching. Depending on age, a thick cartilage cap can lead to an inaccurate registration [28]. In fact, any soft tissue that impedes one’s ability to get to cortical bone, such as tendon insertion, ligaments, articular cartilage, and soft tissue component of tumor can affect the accuracy of the resection due to compromised registration. Picking or creating a mobile segment of bone can lead to inaccurate resection [28], so it is important to appreciate what is mobile or what will be mobile. Once a single bone cut is created a certain amount of uncertainty is introduced into the system for the subsequent cut [2]. Disruption of the ring by ligament sectioning can potentially disturb the accuracy of the spatial relationship and the registration of the patient [2]. The use of navigation can only improve accuracy of bony resection and avoid inadvertent perforation of tumor with osteotome. Navigation is not the perfect system. Narrow soft tissue margins cannot be improved with navigation [22]. It does have several drawbacks including time for surgery [1, 21] due to the registration process [49]. The use of navigation is costly due to surgery time and system itself. It does have a learning curve associated with it but that time lessens as more experience is gained [22]. Although additional operating time is needed for navigation setup, defining the resection plane on preoperatively obtained images can reduce the overall surgical time as no resection margin has to be defined while in surgery [25, 26]. The cumulative accuracy of the entire navigation system can be a limiting factor [18, 20, 37, 58]. Infrared cameras in some studies were felt to be the main contributing factor of inaccuracy [75]. Image fusion involved in CT-MRI fusion is also a type of registration, although the registration is image to image instead of image to patient, process that leads registration error [52, 53]. This is often due to the fact that merging is performed on a visual basis.
22.8 Summary
Sacrum and pelvis three-dimensional anatomy is difficult to understand and master conceptually when trying to resect tumors [51, 76]. Pelvic area surgery is demanding due to anatomy and is difficult to get tumor-free margin leading to higher local recurrence [20, 23, 72, 77]. The purpose of navigation is to provide the surgeon an accurate three-dimensional virtual model of the tumor and pelvic structures including the sacrum that will correlate with the patient’s anatomy at the time of surgery. With this 3D model, the surgeon can plan and map on the navigational software the surgical resection cuts that can be either uniplanar or multi-planar. If needed, although not usually with the sacrum, a custom prosthesis can be made based on the presurgical modeling if standard implants cannot be used. Most importantly, one can choose with the aid of computer navigation the number and vector of the surgical resection as well as a defined margin with respect to the tumor. Navigation serves as a planning tool. Surgery in the sacrum can be hard in normal anatomy and even harder when a tumor distorts the normal anatomy. Computer navigation is relatively new in the musculoskeletal world and has probably been used for tumor resection for less than 10 years. Computer navigation offers a lot of benefits; however, the system is not foolproof. Where computer navigation excels, there is the ability to “see beyond walls.” Working on the sacrum is hard for several reasons; this includes the fact that there are nerves the surgeon would like to spare; it can be hard to identify what level of the sacrum one is at either on intraoperative fluoroscopy or by bony landmarks; and lastly, it is hard to know what lies on the other side of the sacral cut and which direction one is heading. With computer navigation one can literally see where one is heading to in real time and how close ones cut is coming to tumor on the other side. Navigation can only improve bone resection accuracy; it cannot improve the narrow margins associated with soft tissue components due the nature of soft tissue moving because of the inability to navigate soft tissue sarcoma [23, 78].
The future of computer-assisted surgery will include computer navigation but also will begin to include the benefit of robotic-assistive devices and patient-specific instrumentation [72]. Computer navigation is a passive system which only provides information or feedback while the future robotic-assisted surgeries will be performed with a more active system that physically guides and limits the surgeon from straying outside of predetermined resection planes [79]. Currently, systems exist to help with total knee and total hip arthroplasty that provide reproducibility and precision. The benefit to robotic-assisted surgery involves still maintaining control of saws and osteotomes while minimizing the effect of tool vibration and fatigue on part of the surgeons hand [80].
References
1.
Cho HS, et al. Joint-preserving limb salvage surgery under navigation guidance. J Surg Oncol. 2009;100(3):227–32. BACKGROUND: Recently, the navigation system has been introduced to orthopedic oncology. It can apply MRI and/or CT images to intraoperative visualization. We performed navigation-assisted limb salvage surgeries on patients with a malignant bone tumor of the metaphysis of the long bone or the iliac bone while preserving the adjacent joint. METHODS: When preoperative chemotherapy was estimated to be effective by imaging studies and the residual remaining epiphysis was expected to be more than 1 cm long after tumor resection with 1-2 cm of surgical margin, joint-preserving surgery was performed under navigation guidance. We carried out CT and MRI data fusion to use MR images as an intraoperative guide. A deep frozen strut allograft was placed in the defect for the restoration of anatomical continuity. RESULTS: Resection margin measured on pathological examination was in accordance with that of the preoperative plan. The functional scores of all patients were satisfactory. There was no evidence of recurrence on the regional radiographs and CT on the chest until the last follow-up. CONCLUSION: Navigation-assisted surgery can be indicated for limb salvage and it can help to preserve the adjacent joint in selected cases.PubMed
2.
Cho HS, et al. Computer-assisted sacral tumor resection. A case report. J Bone Joint Surg Am. 2008;90(7):1561–6.PubMed
3.
Court C, et al. Surgical excision of bone sarcomas involving the sacroiliac joint. Clin Orthop Relat Res. 2006;451:189–94. Adequate (wide or marginal and uncontaminated) margins and reconstruction are difficult to achieve when performing an internal hemipelvectomy for bone sarcomas involving the sacroiliac joint. We evaluated whether adequate surgical margins could be achieved and if functional outcomes could be predicted based on the type of resection and reconstruction. Forty patients had resections of the sacroiliac joint. Vertical sacral osteotomies were through the sacral wing (n = 2), ipsilateral sacral foramina (n = 27), sacral midline (n = 9), or contralateral foramina (n = 2). Iliac resections were Type I, Type I-II with partial or total acetabular re-section, or Type I-II-III. Surgical margins were adequate in 28 of 38 patients (74%), two (7%) of whom experienced local recurrence, compared with seven of 10 (70%) patients with inadequate margins. Reconstruction consisted of restoring continuity between the spine and pelvis. Resection of the entire acetabulum and removal of the lumbosacral trunk were the two main determinants of function, as assessed using the Musculoskeletal Tumor Society score. There were no life-threatening or function-threatening complications. Internal hemipelvectomy with a limb salvage procedure can be achieved with adequate surgical margins in selected patients. Functional outcomes can be predicted based on the type of resection and reconstruction, which helps the surgeon plan the procedure and inform the patient.PubMed
4.
Fehlberg S, et al. Computer-assisted pelvic tumor resection: fields of application, limits, and perspectives. Recent Results Cancer Res. 2009;179:169–82. The treatment of malignant tumors involving the pelvic area is a challenging problem in musculoskeletal oncology due to the complex pelvic anatomy and the often large tumor size at presentation. The use of navigation systems has effectively increased surgical precision aiming at optimal preservation of pelvic structures without compromising oncologic outcome by means of improved visibility of the surgical field, and enabling intraoperative display and 3D reproduction of preoperatively determined pelvic osteotomy and resection levels. In the following sections, current developments in computer-assisted pelvic surgery are reviewed and possible fields of application, as well as limitations of navigation systems, are discussed.PubMed
5.
Fuchs B, et al. Osteosarcoma of the pelvis: outcome analysis of surgical treatment. Clin Orthop Relat Res. 2009;467(2):510–8. Risk factors to explain the poor survival of patients with osteosarcoma of the pelvis are poorly understood. Therefore, we attempted to identify factors affecting survival and development of local recurrence and metastasis. We retrospectively reviewed 43 patients who had high-grade pelvic tumors and were treated surgically. Twenty lesions were chondroblastic, 10 fibroblastic, 11 osteoblastic, and one each was giant cell-rich and small cell osteosarcomas. At a median of 3.5 years (range, 0.3-21 years) postoperatively, 13 patients were alive with no evidence of disease. The overall and disease-free 5-year survival rates were 38% and 29%, respectively, at 5 years. Anatomic location, tumor size, and margin predicted survival. Fifteen patients (35%) had local recurrence. The 5-year cumulative incidence of recurrence with death as a competing risk factor was 34%. Location in the ilium and size of the tumor predicted local recurrence. Twenty-one (49%) of 43 patients had metastases develop. The cumulative incidence of metastasis with death as a competing risk factor was 48% at 5 years. Six patients who presented with metastasis had a worse survival than patients who had no evidence of metastasis at presentation (2-year survival, 33% versus 76%). If distant metastasis is diagnosed subsequent to primary treatment, aggressive therapy may be justified. LEVEL OF EVIDENCE: Level II, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.PubMed
6.
Kawai A, et al. Osteosarcoma of the pelvis. Oncologic results of 40 patients. Clin Orthop Relat Res. 1998;(348):196–207. The cases of 40 patients with osteosarcoma of the pelvis treated between 1977 and 1994 were reviewed. The location of the tumor was ilium in 30 patients, ischium in four, pubis in one, and sacrum in five. Most (58%) of the tumors were of the chondroblastic subtype. Thirty patients had surgical excision of the tumors: 10 with hemipelvectomies and 20 with limb sparing procedures. A wide margin was achieved in 16 of 30 (53%) patients, including 12 of 14 who had no sacral tumor involvement. Positive margins occurred at the sacrum in 11 patients, lumbar vertebra in one, perirectal space in one, and contralateral pubic body in one. Macroscopic tumor emboli within the regional large vessels were found in seven patients. The incidence of local recurrence was 32%: 13% in wide excisions, 38% in marginal excisions, and 80% in intralesional excisions. The 1- and 5-year overall patient survivals were 73% and 34%, respectively. Patients who had a surgical excision of the primary tumor had a significantly better survival than did those treated without surgery (5-year survival; 41% and 10%, respectively). Tumor size, surgical excision of the primary tumor, surgical margin, and type of surgical procedure were the prognostic factors for patients with Stage IIB tumors.
7.
Pring ME, et al. Chondrosarcoma of the pelvis. A review of sixty-four cases. J Bone Joint Surg Am. 2001;83-A(11):1630–42. BACKGROUND: Treatment of pelvic chondrosarcoma is a difficult problem for the musculoskeletal oncologist. Poor rates of survival and high rates of local recurrence after surgical treatment have been reported in previous studies. The present study was designed to review the long-term oncologic and functional outcomes of surgical management in a large series of patients with pelvic chondrosarcoma who were treated at a single institution. METHODS: The cases of sixty-four patients with localized pelvic chondrosarcoma that had been surgically treated between 1975 and 1996 were reviewed retrospectively. The study was limited to patients who had received no previous treatment for chondrosarcoma. There were forty-one male and twenty-three female patients who had a mean age of forty-seven years (range, fifteen to eighty-eight years). The patients were followed for a minimum of three years or until death. The median duration of follow-up of the living patients was 140 months (range, thirty-nine to 295 months). RESULTS: Thirty-three of the sixty-four patients were first seen with grade-1 chondrosarcoma; twenty-three, with grade-2; one, with grade-3; and seven, with grade-4 (dedifferentiated chondrosarcoma). Thirteen patients had a hemipelvectomy to achieve local tumor control, whereas fifty-one patients underwent a limb-salvage procedure. Twelve patients (19%) had local recurrence, and eleven (17%) had distant metastases. At the time of the final follow-up, forty-four patients (69%) were alive without evidence of disease, thirteen (20%) had died of the disease, six (9%) had died of unrelated causes, and one (2%) was alive with disease. Less than a wide surgical margin correlated with local recurrence (p = 0.014). High-grade tumors correlated with poor overall survival (p < 0.001). All patients who had a limb-salvage procedure were able to walk at the time of the final follow-up, and they had a mean functional score of 77%, according to the system of the Musculoskeletal Tumor Society. CONCLUSIONS: Aggressive surgical resection of pelvic chondrosarcoma results in long-term survival of the majority of patients. There is a high correlation between tumor grade and overall or disease-free survival.PubMed
8.
Sucato DJ, et al. Ewing’s sarcoma of the pelvis. Long-term survival and functional outcome. Clin Orthop Relat Res. 2000;(373):193–201. Fifty patients with Ewing,s sarcoma of the pelvis were treated using a multidisciplinary approach; followup of surviving patients averaged 137 months (range, 40-276 months). The addition of surgical resection to the multidisciplinary treatment for all patients was associated with improved survival compared with survival of patients treated with chemotherapy and radiation therapy alone; the addition of surgery to the treatment regimen of 37 patients without metastases also was associated with improved survival. There were no significant differences between the surgical and nonsurgical groups in terms of tumor size, stage of disease, patient age, duration of symptoms before diagnosis, or anatomic site. Surgery was used more often in recently treated patients, but the year of diagnosis and treatment did not significantly affect overall survival, secondary to large confidence intervals. The Short Form-36 and the Musculoskeletal Tumor Society functional evaluation instruments showed a superior level of function in the nonsurgical group, but this difference was not statistically significant. There have been many advances in the treatment of patients with Ewing’s sarcoma during the past 3 decades, resulting in improved survival for patients with Ewing’s sarcoma of the pelvis. The addition of surgery significantly improved survival and did not show a significant difference in functional outcome.
9.
Wirbel RJ, et al. Surgical treatment of pelvic sarcomas: oncologic and functional outcome. Clin Orthop Relat Res. 2001;(390):190–205. The experiences in treating 93 consecutive patients (56 males, 37 females; mean age, 38.5 years; range, 4-69 years), including 76 patients with primary malignant bone tumors and 17 patients with soft tissue sarcomas involving the innominate bone, are reported. Oncologic and functional results were investigated in relation to the tumor stage, to the achieved surgical margin, and to the surgical procedure (hemipelvectomy, internal hemipelvectomy and endoprosthetic replacement, and continuity resection). The mean followup was 48 months (range, 8-222 months). The 5-year survival was 86% in patients with low-grade malignant bone tumors, 42% in patients with high-grade malignant bone tumors, and 25% in patients with high-grade soft tissue sarcomas. Survival was influenced by the grade of malignancy, the tumor stage, and the achieved surgical margins. Forty-six patients who survived were examined an average of 36 months after primary surgery. Excellent and good functional results were seen in 82% of patients who underwent continuity resection and in 55.5% of patients who underwent partial or total internal hemipelvectomy. All patients who survived hemipelvectomy had poor functional results. Surgical treatment of pelvic sarcomas is an extensive procedure with a considerable incidence of complications. It requires the knowledge of different techniques of resection and reconstruction of bone, joints, soft tissue, and intrapelvic organs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree