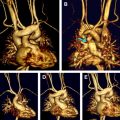
In neonates and infants with congenital heart disease (CHD), cardiovascular magnetic resonance (CMR) is an established imaging modality in all patients in whom echocardiography does not provide sufficient information and definitive diagnosis. CMR is noninvasive, and does not involve vascular catheterization or ionizing radiation. Therefore the use of CMR obviates the potential risks of cardiac catheterization in critically ill infants. This article discusses the use of CMR in newborns with CHD before cardiac surgery, focusing on conotruncal anomalies, pulmonary venous anomalies, complex CHD in visceroatrial heterotaxy, borderline hypoplastic left heart syndrome, and the use of contrast medium in newborns.
In neonates and infants with congenital heart disease (CHD), cardiovascular magnetic resonance (CMR) is an established imaging modality in all patients in whom echocardiography does not provide sufficient information and definitive diagnosis.
CMR is nowadays a well-used alternative to angiocardiography because of its noninvasiveness, and the noninvolvement of vascular catheterization or ionizing radiation. Therefore the use of CMR obviates the potential risks of cardiac catheterization in critically ill infants ; cardiac catheterization in newborns is limited to catheter-guided interventions, such as atrial septectomy or ductal stenting.
This article discusses the use of CMR in newborns with CHD before cardiac surgery. As imaging of the extracardiac vasculature is discussed in another article in this issue, the authors focus here on conotruncal anomalies, pulmonary venous anomalies, complex CHD in visceroatrial heterotaxy, and borderline hypoplastic left heart syndrome. One separate section is dedicated to the use of contrast medium in newborns.
Conotruncal anomalies
Conotruncal anomalies are the result of an embryologic septation defect of the conotruncus and include interrupted aortic arch, truncus arteriosus communis, tetralogy of Fallot, pulmonary atresia, double-outlet right ventricle, transposition of the great arteries and ventricular septal defect associated with septal malalignment and/or aortic arch anomalies. Conotruncal anomalies represent the most frequent indication for performing preoperative CMR in newborns. Interruption of the aortic arch and pulmonary blood supply in pulmonary atresia are presented by Krishnamurthy and Lee in another article in this issue.
In general, in conotruncal anomalies axial images acquired at the level of the cardiac basis can provide a first pictorial orientation showing the position of the great arteries within the heart and to each other, and provide an initial clue to the diagnosis of these defects ( Fig. 1 ).

Truncus arteriosus communis
Background
Truncus arteriosus communis results from the failure of the conotruncus to divide into a separate aorta and pulmonary artery. Thus characteristic of this anomaly is a single large vessel (truncus) that arises from the midline base of the heart (see Fig. 1 ) and divides into the systemic, pulmonary, and coronary circulations. A ventricular septal defect located below the truncus always occurs. The classification of the different types (I–IV) of the disease is based on the description of the anatomy of the pulmonary arteries, as shown in Fig. 2 . The truncal valve can be thickened or dysplastic and present tricuspid in 40% – 60%, quadricuspid in 25% – 30% and bicuspid in 10% – 30% of the patients. Stenosis or regurgitation of the truncal valve is not uncommon. Anomalies of the pulmonary arteries occur rarely; however, isolated origin of a pulmonary artery from a duct or from an aortopulmonary collateral, absence of one pulmonary artery, stenosis or hypoplasia, and crossed or malpositioned pulmonary arteries have been described. The aortic arch is right-sided in about one-third of the patients. Aortic coarctation or interrupted aortic arch is found in 15% to 20% of the cases. Finally, anomalies of the coronary arteries may present with variable patterns of origin that are independent from the number of truncal leaflets.
Surgical correction for truncus arteriosus communis consists of ventricular septal defect (VSD) closure and insertion of a valved conduit between the right ventricle and the pulmonary arteries. Depending on the type of truncus arteriosus, the conduit can be sutured directly to the pulmonary trunk, or the complete pulmonary bifurcation can be reconstructed. In addition, reconstruction of the truncal valve may be required.
Preoperative Imaging
The following structures need to be carefully assessed by cardiac imaging before surgery:
- •
Pulmonary arteries
- •
VSD
- •
Truncal valve
- •
Ascending aorta and the aortic arch
- •
Coronary arteries.
Even if the primary anatomy can usually be defined by neonatal echocardiography, demonstration or exclusion of all potentially associated anomalies may require additional imaging. A stack of axial images, acquired with the spin echo (SE) or the steady-state free precession (SSFP) sequence, demonstrate a single large vessel arising from the midline base of the heart, and the pulmonary arteries, with or without a main stem, originating directly from the common trunk ( Fig. 3 ). If several thin slices are acquired parallel to the plane of the truncal valve, the cusps of the truncus valve may be visualized. Contrast-enhanced MR angiography (CEMRA) accurately defines the exact anatomy of the pulmonary arteries and of the aortic arch and its branches ( Fig. 4 ). Velocity-encoded phase-contrast imaging (PC cine) can be used for exact quantification of the regurgitant volume and fraction of an insufficient truncal valve.
Truncus arteriosus communis
Background
Truncus arteriosus communis results from the failure of the conotruncus to divide into a separate aorta and pulmonary artery. Thus characteristic of this anomaly is a single large vessel (truncus) that arises from the midline base of the heart (see Fig. 1 ) and divides into the systemic, pulmonary, and coronary circulations. A ventricular septal defect located below the truncus always occurs. The classification of the different types (I–IV) of the disease is based on the description of the anatomy of the pulmonary arteries, as shown in Fig. 2 . The truncal valve can be thickened or dysplastic and present tricuspid in 40% – 60%, quadricuspid in 25% – 30% and bicuspid in 10% – 30% of the patients. Stenosis or regurgitation of the truncal valve is not uncommon. Anomalies of the pulmonary arteries occur rarely; however, isolated origin of a pulmonary artery from a duct or from an aortopulmonary collateral, absence of one pulmonary artery, stenosis or hypoplasia, and crossed or malpositioned pulmonary arteries have been described. The aortic arch is right-sided in about one-third of the patients. Aortic coarctation or interrupted aortic arch is found in 15% to 20% of the cases. Finally, anomalies of the coronary arteries may present with variable patterns of origin that are independent from the number of truncal leaflets.
Surgical correction for truncus arteriosus communis consists of ventricular septal defect (VSD) closure and insertion of a valved conduit between the right ventricle and the pulmonary arteries. Depending on the type of truncus arteriosus, the conduit can be sutured directly to the pulmonary trunk, or the complete pulmonary bifurcation can be reconstructed. In addition, reconstruction of the truncal valve may be required.
Preoperative Imaging
The following structures need to be carefully assessed by cardiac imaging before surgery:
- •
Pulmonary arteries
- •
VSD
- •
Truncal valve
- •
Ascending aorta and the aortic arch
- •
Coronary arteries.
Even if the primary anatomy can usually be defined by neonatal echocardiography, demonstration or exclusion of all potentially associated anomalies may require additional imaging. A stack of axial images, acquired with the spin echo (SE) or the steady-state free precession (SSFP) sequence, demonstrate a single large vessel arising from the midline base of the heart, and the pulmonary arteries, with or without a main stem, originating directly from the common trunk ( Fig. 3 ). If several thin slices are acquired parallel to the plane of the truncal valve, the cusps of the truncus valve may be visualized. Contrast-enhanced MR angiography (CEMRA) accurately defines the exact anatomy of the pulmonary arteries and of the aortic arch and its branches ( Fig. 4 ). Velocity-encoded phase-contrast imaging (PC cine) can be used for exact quantification of the regurgitant volume and fraction of an insufficient truncal valve.
Tetralogy of Fallot
Newborns with uncomplicated native tetralogy of Fallot (TOF) can be usually completely assessed by echocardiography. However, in severe forms with extremely diminutive pulmonary arteries, advanced anatomic imaging is essential for correct planning of a tailored treatment strategy, which usually begins with a palliative intervention and eventually ends with surgical repair. CMR, and particularly CEMRA, can be performed before palliation for visualization of the right ventricular outflow tract, main pulmonary artery, and the side branches. The anatomic findings described will determine the choice of the appropriate palliation procedure, such as surgical Blalock-Taussig shunt or a catheter-guided stenting of the ductus arteriosus, or stenting of the right ventricular outflow tract. In the presence of a dual lung supply, when lung perfusion is warranted by both the native pulmonary arteries and aortopulmonary collateral arteries, catheter-guided coiling of selected aortopulmonary collaterals at the time of palliation increases flow into the native pulmonary arteries and therefore their growth.
During follow-up, CMR is the ideal tool for documenting growth of the pulmonary arteries and for planning surgical repair or other interventions. The accuracy of measurements of the vessel size on CEMRA images has been previously demonstrated even for vessels as small as 2 mm ( Fig. 5 ).
Such individually tailored management with a staged treatment approach, supported by repeated imaging of the pulmonary arteries, may eventually result in a successful surgical repair.
Double-outlet right ventricle
Background
Double-outlet right ventricle (DORV) is defined by a ventriculoarterial connection in which both great arteries arise completely or predominantly from the morphologically right ventricle (see Fig. 1 ). The spatial relationship of the great arteries to each other shows a wide spectrum, ranging from a normal position, to a side-by-side position, to a transposed position. In DORV the ejected stroke volume of the left ventricle passes though a VSD, which is an integral part of the anomaly. On the basis of its spatial relationship to the great arteries, the VSD is classified as subaortal, subpulmonal, doubly committed, or remote. Additional anatomic findings potentially complicating management include total atrioventricular septal defect (particularly in right atrial isomerism), straddling of the atrioventricular valves, restrictive VSD, multiple VSD, some degree of ventricular hypoplasia, and obstructive anomalies of the aortic arch. DORV has been frequently observed in hearts with right atrial isomerism.
Preoperative Imaging
The diagnosis of DORV is usually done by echocardiography. Initially the defect may be “naturally” palliated, if a pulmonary stenosis is present, or require palliation with a banding of the pulmonary artery or an aortopulmonary shunt. Surgical repair is then performed some months later. The primary aim of surgical correction is to direct blood flow from the left ventricle through the VSD into the aorta. Accurate definition of the size and position of the VSD within the heart and of its relation to the aorta is crucial for planning surgical repair. Beekmana and colleagues and Yoo and colleagues demonstrated the usefulness of cross-sectional CMR for describing the pertinent features of VSD in DORV. By using transverse planes they found that the site of fusion of the outlet septum with the VSD margin was the most important diagnostic feature for differentiating subaortic from subpulmonal VSD; fusion was absent in doubly committed VSD. Similarly, Beekmana and colleagues showed that CMR can accurately assess the spatial relationship between the semilunar valves and the VSD as well as the morphology of both outflow tracts ( Fig. 6 ). In 30% of the cases CMR provided additional information compared with conventional imaging. By contrast, CMR was not reliable in visualizing aberrant chordae tendinae, or straddling of the atrioventricular valves. In addition to intracardiac imaging, CEMRA and its 3-dimensional (3D) reconstructed images complete the anatomic information by showing the extracardiac venous and arterial anatomy in all its possible variances.
Transposition of the great arteries
Complete Transposition of the Great Arteries
Transposition of the great arteries (TGA) can be entirely diagnosed by echocardiography. The most common associated anomalies include a VSD, left ventricular outflow tract obstruction, atrial septal defect, patent ductus arteriosus, and anomalies of the aortic arch such as right aortic arch, aortic coarctation, and double aortic arch. The origins of the coronary arteries are widely variable and need to be exactly described preoperatively, as during the arterial switch operation the surgeon will transfer the coronary arteries into the neo-aorta.
Advanced imaging is rarely required preoperatively, and occurrence of TGA in more complex CHD is the main indication for performing CMR in these patients ( Fig. 7 ).
Congenitally Corrected Transposition of the Great Arteries
Congenitally corrected TGA (cTGA) is an entity characterized by atrioventricular and ventriculoarterial discordance. The right atrium is connected to the left ventricle and the left atrium is connected to the right ventricle. The aorta arises from the right ventricle and the pulmonary artery from the left ventricle (see Fig. 1 ). The aorta is located anteriorly and to the left of the pulmonary artery ( Fig. 8 ). This defect is called corrected transposition of the great arteries as, unlike in TGA, which is a cyanotic CHD, in cTGA the normal hemodynamic pathways are maintained.
Recognition of cTGA at echocardiography can be challenging. Indeed cross-sectional imaging may facilitate the segmental approach to cardiac anatomy. SSFP axial images show the situs, the position of the heart, the systemic and pulmonary venous drainage, the morphology of both atria and both ventricles, and the origin of the great arteries; the connections among the cardiac segments can be accurately analyzed and defined ( Fig. 9 ). If needed, subsequent oblique images can be helpful in further delineating the segmental connections. Finally, CEMRA enables recognition of additional cardiac and extracardiac findings.
Additional malformations in cTGA are common :
- •
VSD (70%)
- •
Pulmonary valve stenosis (30%–50%)
- •
Dysplasia of the tricuspid valve (with Ebstein)
- •
Dextrocardia (20%)
- •
Situs inversus (5%–8%)
- •
Anomalies of the conduction system with high risk for complete heart block.
More rarely associated anomalies include superior/inferior position of the ventricles, aortic coarctation, interruption of the aortic arch, hypoplasia of one ventricle, common arterial trunk, and straddling or overriding of the left atrioventricular valve.
Anomalies of the pulmonary veins
Background
Anomalous pulmonary venous connection is characterized by one or more pulmonary veins connecting to either the right atrium or a systemic vein, or to both. If all 4 pulmonary veins connect anomalously, they usually form a confluence behind the left atrium. This additional vascular structure behind the left atrium can be seen prenatally by fetal echocardiography, and represents one of the clues to the diagnosis of anomalous pulmonary venous connection in the fetus. Total anomalous pulmonary venous connection (TAPVC) is classified as cardiac (27% of cases), supracardiac (51%), or infracardiac (15%) type, depending on the site of drainage ( Fig. 10 ). A mixed type of TAPVC (7%) is present if part of the pulmonary veins connects at one level and part to another. In supracardiac or infracardiac type, a pulmonary venous channel, the so-called vertical vein, runs cranially, to drain into the superior systemic vein system, or caudally, crossing the diaphragm to drain into the infradiaphragmatic venous system ( Fig. 11 ). Obstruction may occur at different levels within the course of the venous pathway ( Box 1 ). Cardiac type and mixed type are often associated with more complex CHD, particularly visceroatrial heterotaxy.
Stenosis of the individual pulmonary vein
Stenosis at the site of abnormal connection
Intrinsic stenosis of the vertical vein
Extrinsic compression of the vertical vein
Anatomic vice (supracardiac type): compression of the vertical vein between the bronchus and the pulmonary artery
Small esophageal hiatus in infracardiac type
Portal vein-ductus venosus complex in the liver (infracardiac type)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree