Owing largely to advances in fetal echocardiography, in most developed countries the diagnosis of severe congenital heart disease (CHD) is now made during gestation, and delivery is electively planned in hospitals that have the facilities and expertise to manage these patients, with magnetic resonance (MR) imaging performing an important complementary role. MR imaging as a sole imaging modality for comprehensive presurgical evaluation is also increasingly being explored. This article focuses on the imaging of neonatal CHD by MR, followed by a brief discussion of the safety of gadolinium-based contrast agents in this age group.
Not too long ago, the perinatal period was fraught with peril for fetuses with severe congenital heart disease (CHD). Neonates presented in a critical condition at birth or in the first week of life, usually following ductal closure. Their survival was diminished by the lack of trained personnel and specialized infrastructure at the place of delivery, and by the emergent nature of the palliative intervention. Owing largely to advances in fetal echocardiography, in most developed countries the diagnosis of severe CHD is now made during gestation, and delivery is electively planned in hospitals that have the facilities and expertise to manage these patients. In the vast majority of cases, postnatal chest radiography and echocardiography (echo) are often the only diagnostic modalities needed for preoperative planning before initial palliation. When echo cannot provide a comprehensive picture of relevant cardiovascular anatomy, MR imaging performs an important complementary role, with diagnostic catheterization being restricted to a small number of patients who need clarification of complex coronary anatomy, or measurement of chamber pressures and oxygen saturation. The role of MR imaging as a sole imaging modality for comprehensive presurgical evaluation is also increasingly being explored for conditions that have complex multisystem involvement, such as heterotaxy, or associated neurologic involvement that must be evaluated before surgery. This review focuses on the imaging of neonatal CHD by MR, followed by a brief discussion of the safety of gadolinium-based contrast agents in this age group.
Neonatal cardiac MR imaging technique
Neonatal cardiovascular MR imaging is one of the most difficult imaging challenges in pediatrics, due to the adaptation needed for the complex structural changes in severe CHD, the small size of the structures of interest, rapid heart rate and breathing rate, altered hemodynamics, and the relatively high risk of sedating these sick neonates. It is a dynamic user-dependent examination, with changes to the protocol being needed based on real-time evaluation of the initial sequences.
Patient Preparation
Before the start of an MR examination in a neonate with CHD, review of the chest radiograph helps to confirm position of the heart in the thorax, which will determine positioning of the electrocardiography (ECG) electrodes. Robust synchronization with the ECG signal using vectorcardiogram triggering as well as the use of respiratory bellows for pulse sequences needing respiratory triggering are part of the standard preparation.
Intravenous Access
Because the first-pass MR angiography is the gold standard for evaluation of the extracardiac vasculature, selecting an appropriate route of contrast administration is critical to the success of the study. In the immediate postnatal period, intravenous access in the arm or leg is preferred to an umbilical venous catheter, which could potentially terminate in an occluded ductus venosus. A 22-gauge or 24-gauge needle is used for intravenous access, with injection rates of 1 to 2 mL per second.
Sedation
Breath-holding will reduce motion blurring, and yield sharper and potentially more diagnostic images. However, breath-holding requires general endotracheal anesthesia (GETA) with controlled ventilation. In the authors’ experience, most pulse sequences can be performed in sedated and freely breathing patients to yield diagnostic images, with GETA being restricted to evaluation of very small vascular structures such as aortopulmonary collaterals.
Coil Selection
Phased-array coils are preferred because they allow for the use of parallel imaging techniques, which significantly reduce scan time. A phased-array receive coil that provides adequate coverage should be used. The coverage should extend from the middle of the neck to the level of the renal vessels in all patients, so that important findings outside the thorax such as infradiaphragmatic total anomalous pulmonary venous return (TAPVR) or unusual branching patterns of the head and neck vessels can be identified. Ideal anatomic coverage is provided by the head or shoulder coil in neonates. Newer multipurpose coils such as the head and neck coil or the neurovascular coil are good choices if combined evaluation of the head and cardiovascular system is desired. Dedicated neonatal phased-array cardiac and body coils are becoming increasingly available, providing an excellent signal with high spatial resolution. MR imaging–compatible incubator/coil combinations have also been introduced, which regulate temperature, humidity, and oxygen concentration while providing monitoring capabilities during transport and scanning for these critical neonates with tenuous hemodynamic and metabolic status.
Pulse Sequences
All sequences have benefited from the advent of parallel imaging, which uses information from multiple coil elements to speed up acquisition or increase temporal resolution. The following is a summary of pulse sequences used for neonatal imaging.
Black-blood sequences
The most commonly used sequences are fast spin echo double-inversion recovery sequence, performed with ECG gating and breath-holding, or a spin echo echoplanar imaging sequence, performed with ECG gating and free breathing. In the free-breathing sequences, respiratory motion is compensated by multiple signal averages with or without respiratory triggering. Black-blood sequences provide multislice static images, with excellent spatial resolution even in neonates. These sequences provide an overview of vascular anatomy, spatial chamber relationships, airway morphology, and abdominal visceral anatomy.
Bright-blood sequences
The most commonly used cine sequence is segmented k-space steady-state free precession (SSFP). It has excellent temporal resolution, allowing for multiphase evaluation across the cardiac cycle, and optimal myocardial and blood pool contrast. However, cine SSFP is limited by out-of-plane flow-related phase incoherence artifact, especially in small patients with rapid flow. In addition, the spatial resolution achievable in cine SSFP is limited. Therefore, the old “workhorse” sequence, cine fast gradient echo (cine GRE) with segmented k-space sequence, is preferred to the SSFP sequence when thin slices with high resolution are required in neonates. Cine GRE can be acquired with free breathing and multiple signal averaging. It is more sensitive to in-plane intravoxel dephasing signal loss from flow turbulence, which can be used as an important diagnostic clue to the presence of shunts, stenosis, and regurgitation. In the neonatal period, the cine GRE sequence is frequently performed in the axial plane with overlapping thin slices, to track the course of the extracardiac vasculature and to determine venoatrial connections.
Alternatively, isotropic whole heart coverage using a 3-dimensional (3D) SSFP sequence with respiratory navigator gating has been used to provide comprehensive static, high-resolution morphologic bright-blood evaluation of intracardiac morphology, coronary anatomy, and extracardiac vascular anatomy in one sequence. It can serve as an alternative (or backup) angiographic data set, and is not subject to the vagaries of distribution of intravenous contrast. However, this technique is not available on all magnet platforms, and is not as robust in small children or in those with high heart rates. Tips to increase the quality of 3D SSFP imaging in neonates with high heart rates include using high spatial resolution (<1 mm isotropic), small respiratory navigator window (2–3 mm), small shot duration (50–70 milliseconds), timing the acquisition to end-systole, low parallel imaging factor, and dedicated phased-array coils with a larger number of elements.
Flow velocity mapping is accomplished by a gradient echo–based pulse sequence known as phase contrast (PC). PC imaging can be used to quantify stroke volume, valvular regurgitation, Qp:Qs, differential pulmonary artery flow, venous return, coronary flow, and pressure gradients across stenoses using the modified Bernoulli equation. There is usually little role for PC imaging in the preoperative setting in neonates.
MR angiography
The most common sequence used is contrast-enhanced MR angiography (CEMRA) performed in dynamic fashion following bolus injection of gadolinium using a 3D T1-weighted fast gradient echo sequence. CEMRA provides a high-resolution 3D data set, with its main application being evaluation of the extracardiac thoracic vasculature, including the pulmonary arteries, pulmonary veins, aorta, systemic veins, and collateral blood supply to the lungs. CEMRA may be performed in a time-resolved fashion even in neonates with high heart rates by incorporating k-space undersampling techniques such as keyhole, with parallel imaging techniques such as SENSE (sensitivity encoding) and centric encoding of k-space to achieve highly accelerated dynamic times of up to 1 to 2 seconds while preserving spatial resolution ( Fig. 1 ). When used as a time-resolved technique, MR angiography also provides the added advantage of reliable first-pass imaging, which is independent of timing of contrast injection or acquisition, and dynamic information, which provides insights into the hemodynamics of the disease process. Techniques of postprocessing include multiplanar reformatting, maximum-intensity projection, volume rendering, and virtual endoscopy.

Imaging planes
Black-blood imaging is performed in the axial, oblique sagittal, or coronal planes, with the latter being aligned along the trachea and proximal bronchi. Bright-blood imaging is most commonly performed in the axial plane to track the course of the extracardiac vasculature. Vertical long-axis, 4-chamber, and short-axis planes are also routinely used, with right ventricular and left ventricular outflow tract views, aortic root short-axis views, with customized planes to image the aortic arch or pulmonary arteries being used whenever necessary. 3D MR angiograms are performed in the sagittal or coronal planes.
Protocols and indications for neonatal cardiac MR imaging
Initial palliation stands for first-stage surgical procedures that are typically done immediately after birth to stabilize the patient, including relieving pulmonary vein, pulmonary artery, or aortic outflow obstruction, augmentation of pulmonary blood flow with a modified Blalock-Taussig (BT) shunt, or reducing pulmonary blood flow with a pulmonary artery band. Echocardiography is an excellent tool in expert hands, providing most of the information required for planning initial palliation. However, it may be limited in some situations because of a narrow field of view, lack of acoustic windows, and inability to delineate complex spatial relationships and the extracardiac vascular anatomy, especially pulmonary veins, systemic veins, aortic arch, and arterial collaterals. MR imaging is complementary in this setting, providing a high-resolution 3D data set, with excellent delineation of complex spatial relationships and the extracardiac vascular anatomy with a high degree of diagnostic confidence. In a study comparing MR imaging with echocardiography and cardiac catheterization in presurgical planning of heterotaxy, accurate delineation of pulmonary venous connections was not achieved by echocardiography in 42% of patients, and by catheterization in 25% of patients. The interobserver agreement was also better on MR imaging relative to echocardiography or catheterization.
A short and efficient MR imaging study is paramount in neonatal patients because neonatal patients with severe CHD are potentially unstable. A comprehensive evaluation is neither desirable nor feasible. The imaging question for MR imaging is discrete, frequently pertaining to the status of the pulmonary and systemic vasculature. Therefore, the MR imaging protocol is usually limited to axial and oblique cine GRE sequence performed with 3- to 4-mm slice thickness with overlap to diminish errors related to volume averaging, thin-section black-blood images in the axial and/or oblique planes through the chest, and a high-resolution gadolinium-enhanced 3D MR angiogram. Functional/flow/myocardial evaluation is not routinely performed unless it provides important diagnostic or prognostic information that is not available on echo.
A discussion of common indications for neonatal cardiac MR imaging follows, with suggestions on protocol modifications based on indication:
Evaluation of Cardiac Morphology
Echo is quite successful in delineating intracardiac morphology and function in this age group, including atrial, ventricular, and great arterial relationships, valvular status, and ventricular function, and is aided by the liberal acoustic windows and the ability to use high-resolution transducers. There are only a few indications for MR imaging or computed tomography (CT) for determining intracardiac morphology, and they involve rare entities with complex segmental anatomy, alterations of the chest wall that affect acoustic windows, or entities that require rigorous quantitation of morphologic and functional parameters for decision making regarding management. Examples include:
- •
Clarification of unusual or complex segmental cardiac anatomy, as in some types of single ventricle ( Fig. 2 ), criss-cross atrioventricular relationships, ectopia cordis, and conjoined twins with thoracopagus
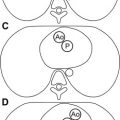
Fig. 2
MR imaging for evaluation of cardiac morphology. A newborn with {S,L,L} transposition of great arteries and tricuspid atresia. MR imaging was performed to assess the status of the aortic arch. ( A ) Four-chamber view of a cine fast gradient echo (cine GRE) sequence showing L-looped ventricles, hypoplastic right ventricle ( white arrow ), and tricuspid atresia ( black arrow ). LV, left ventricle. ( B ) Cranial to A , showing restrictive bulboventricular foramen ( asterisk ), leading to a hypoplastic infundibular outlet chamber, and pulmonary artery (Pa) arising from the left ventricle. ( C ) Cranial to B , showing L-malposed aorta (Ao) arising from the infundibular outlet chamber. ( D ) Oblique sagittal cine GRE image showing diffuse hypoplasia of the aortic arch ( arrow ) caused by proximal obstruction at the level of the bulboventricular foramen.
- •
In the setting of cardiac tumors, to determine relationship to inflow and outflow pathways, and for tissue characterization
- •
For decision making regarding single-ventricle or two-ventricle therapy in patients with borderline left ventricular hypoplasia, or double-outlet right ventricle
- •
Assessment of myocardial perfusion and viability in patients with anomalous origin of the coronary artery from the pulmonary artery.
Systemic Evaluation in the Setting of Cardiovascular Disease
There is growing recognition of the fact that CHD is a systemic disease, with cardiovascular, neurologic, and visceral involvement and/or sequelae. Therefore, MR imaging has been used as a “one-stop shop” for neonates with severe CHD to evaluate systemic involvement. MR offers several advantages in this setting, including large field of view, 3D data set with unlimited planes of evaluation, excellent soft-tissue contrast, ability to screen for acute changes like intracranial ischemia and hemorrhage, and screening for stigmata of associated syndromes ( Fig. 3 ).
- •
MR imaging has been used to evaluate the entire spectrum of visceral, cardiac, and vascular abnormalities in heterotaxy in an efficient and accurate manner in comparison with echo. The protocol comprises black-blood imaging performed in the coronal plane, aligned along the trachea and proximal bronchi, a rapid single-shot fast spin echo T2-weighted axial sequence through the abdomen to evaluate the abdominal manifestations of heterotaxy, an axial cine GRE bright-blood sequence with thin, overlapping slices to track the course of the systemic and pulmonary vessels, and gadolinium-enhanced MR angiography ( Fig. 4 ).