Airspace consolidation is defined as replacement of gas within the airspaces by fluid, protein, cells, or other material. Consolidation is characterized on radiographs and computed tomography (CT) by the presence of one or more fairly homogeneous opacities associated with obscuration of the pulmonary vessels and little or no loss of volume. Homogeneous opacities that do not obscure the underlying pulmonary vessels are referred to as ground-glass opacities. Although ground-glass opacities may result from partial filling of the airspaces, they frequently reflect the presence of predominantly interstitial abnormalities and are therefore discussed in Chapter 5 .
The differential diagnosis of airspace consolidation must take into consideration the pattern and distribution of the consolidation, the presence of associated findings such as lymph node enlargement, patient age, recent travel history, and symptoms such as fever or hemoptysis. The differential diagnosis should consider the various substances that may fill the airspaces, namely, water (e.g., edema), blood (e.g., pulmonary hemorrhage), pus (e.g., pneumonia), cells (e.g., adenocarcinoma, lymphoma, organizing pneumonia), fat (e.g., lipoid pneumonia), or protein (e.g., alveolar proteinosis). Although in the vast majority of cases consolidation seen radiologically reflects airspace filling, consolidation may occasionally result from encroachment of the airspaces by extensive interstitial disease, as may be seen, for example, in some patients with sarcoidosis. Furthermore, several conditions classified as interstitial lung diseases, such as acute interstitial pneumonia and organizing pneumonia, have a major airspace component histologically and radiologically. Although it has been suggested that the terms airspace filling or airspace shadowing are preferable to consolidation, these terms are seldom used in clinical practice.
In most cases the differential diagnosis of parenchymal consolidation is based on a combination of clinical, laboratory, and chest radiographic findings. CT is performed mainly in patients with suspected underlying disease (e.g., endobronchial tumor, bronchiectasis, pulmonary thromboembolism) or complications (e.g., abscess formation, empyema) and in patients with progressive consolidation who are not responding to therapy. In this chapter we consider the radiographic and CT findings and differential diagnosis of consolidation.
Chest Radiograph
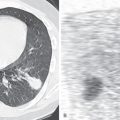
Consolidation is characterized on radiographs by the presence of one or more fairly homogeneous opacities associated with obscuration of the pulmonary vessels and little or no loss of volume ( Fig. 2.1 ). The margins of the consolidation are usually poorly defined except where the consolidation abuts the pleura, such as a fissure. The poor definition of the margins results from partial filling of the airspaces as the consolidation spreads to involve adjacent normal lung. Air-containing bronchi (air bronchograms) are frequently visible within the consolidation. Air bronchograms may not be seen if there is complete bronchial obstruction (e.g., distal to a tumor) or filling of the bronchi with blood (e.g., pulmonary infarction) or inflammatory secretions and pus (e.g., bronchopneumonia).

Silhouette Sign
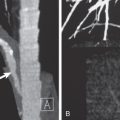
The mediastinal and diaphragmatic contours are normally seen on the chest radiograph where they abut contiguous air-containing lung. When the consolidation is situated in any portion of lung adjacent to a mediastinal or diaphragmatic border, that border can no longer be seen radiographically. Obscuration of the silhouette of the heart, aorta, or diaphragm by an adjacent opacity is known as the silhouette sign (see Fig. 2.1 ). The corollary is that an opacity within the lungs that does not obliterate the mediastinal or diaphragmatic contour cannot be situated within lung contiguous to these structures. Although the silhouette sign is most useful in the differentiation of middle lobe and lingular disease (which obscures the silhouette of the adjacent heart) from lower lobe disease (which obscures the silhouette of the adjacent diaphragm), it may also provide precise anatomic information in other sites. Such information includes obliteration of the diaphragm by consolidation in the adjacent lower lobe ( Fig. 2.2 ), obliteration of the aortic arch on the left side by airlessness of the apicoposterior segment of the left upper lobe, obliteration of the ascending arch of the aorta and the superior vena cava by consolidation of the anterior segment of the right upper lobe, and obliteration of the posterior paraspinal line by contiguous airless lung in the left posterior gutter. Assessment of the silhouette sign is reliable only on radiographs performed with proper technique. Underpenetration may result in loss of visibility of a normal border. Another potential pitfall is the presence of pectus excavatum, which may result in obscuration of the right heart border because of replacement of aerated lung along the right cardiac border by the depressed thoracic wall ( Fig. 2.3 ).


Focal and Multifocal Consolidation
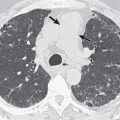
Consolidation may be focal, patchy, or distributed widely throughout both lungs. Focal consolidation may be segmental or nonsegmental in distribution; occasionally, it involves an entire lobe or lung. Segmental consolidation with or without associated volume loss typically results from endobronchial obstruction (e.g., pulmonary carcinoma) or from pulmonary infarction (e.g., thromboembolism or angioinvasive aspergillosis). A segmental distribution can also be seen after aspiration and with pneumonia caused by Staphylococcus aureus, Streptococcus pyogenes, or a variety of gram-negative bacteria. These organisms more commonly cause multifocal or patchy bilateral consolidation (bronchopneumonia), however ( Fig. 2.4 ). A similar distribution may be seen in severe fungal pneumonia, particularly in immunocompromised patients. Lobar (nonsegmental) consolidation is most commonly due to pneumonia, typically secondary to Streptococcus pneumoniae or Klebsiella pneumoniae, in which case it is associated with air bronchograms and normal (see Fig. 2.1 ) or, occasionally, increased lung volume (lobar expansion). Lobar expansion results in convexity of the interlobar fissure (bulging fissure sign) ( Fig. 2.5 ). Less commonly, lobar or segmental consolidation is secondary to bronchial obstruction (e.g., pulmonary carcinoma), in which case it is typically associated with atelectasis and a lack of air bronchograms.


Parenchymal consolidation may also result in poorly defined 5- to 10-mm nodular opacities known as airspace nodules ( Fig. 2.6 ). They have been shown to represent involvement of respiratory bronchioles and surrounding alveoli and are particularly common in patients with infectious bronchiolitis and early bronchopneumonia. These nodular opacities have a centrilobular distribution and are more commonly seen on high-resolution CT than on radiographs.

Spherical (round) areas of consolidation may occur in pneumonia (“round pneumonia”) ( Fig. 2.7 ), septic embolism, focal organizing pneumonia, atelectasis (“round atelectasis”), and neoplasms (particularly adenocarcinoma and lymphoma), as well as occasionally in the early phases of acute respiratory distress syndrome (ARDS). Round pneumonia occurs much more frequently in children than in adults. Although round pneumonia in adults may result from bacterial infection (particularly S. pneumoniae and Haemophilus influenzae ), most commonly no organism is identified. Occasionally, round pneumonia may result from viral infection (e.g., severe acute respiratory syndrome [SARS] caused by coronavirus) or from Q fever, a zoonosis caused by Coxiella (Rickettsia) burnetii. Patients with round pneumonia usually have acute to subacute symptoms of community-acquired pneumonia. However, some patients may be asymptomatic or exhibit nonspecific symptoms. Because most cases of round pneumonia are easily treated with antibiotics, this diagnosis should be considered in all patients with a rounded area of consolidation. Focal organizing pneumonia is a distinct entity that may result from incomplete or delayed resolution of bacterial, viral, or fungal pneumonia, or it may be idiopathic. The histologic features include a chronic inflammatory infiltrate and granulation tissue polyps within the airspaces. Round areas of consolidation that grow slowly over a period of several months should suggest the diagnosis of pulmonary carcinoma or lymphoma.

Occasionally, focal areas of consolidation may be due to pulmonary edema. This is seen most typically with myocardial infarction, resulting in papillary muscle dysfunction or rupture. Affected patients have acute mitral regurgitation toward the orifice of the right superior pulmonary vein, causing preferential distribution of edema to the right upper lobe. The consolidation in these patients is typically nonsegmental and may range from mild, predominantly perihilar upper lobe consolidation to dense consolidation involving the entire right upper lobe. Less common causes of focal or multifocal consolidation include pulmonary vein occlusion, edema in patients with extensive pulmonary vascular obstruction (e.g., severe acute pulmonary embolism resulting in edema in regions of lung with less extensive disease), and reexpansion pulmonary edema. Reexpansion pulmonary edema is an iatrogenic complication that occurs after rapid reexpansion of a collapsed lung following drainage of pneumothorax or hydrothorax ( Fig. 2.8 ). It typically appears suddenly within 1 hour after lung reexpansion. The process usually involves the entire reexpanded lung, although on rare occasions only a single lobe or segment may be involved. In most cases reexpansion pulmonary edema increases in severity for 24 to 48 hours and then slowly resolves over the next 5 to 7 days.

Hemorrhage should be considered, particularly in patients with hemoptysis and in those with blunt chest trauma. Causes of focal pulmonary hemorrhage and hemoptysis include pulmonary carcinoma, bronchiectasis, pulmonary embolism, and infarction. Causes of multifocal or diffuse areas of hemorrhage include granulomatosis with polyangiitis (formerly Wegener granulomatosis), Goodpasture syndrome, microscopic polyangiitis, and systemic lupus erythematosus ( Fig. 2.9 ).

Multifocal consolidation is most commonly due to viral, bacterial, or fungal bronchopneumonia (see Fig. 2.4 ). The consolidation may be unilateral or bilateral.
Chronic conditions typically associated with multifocal consolidation include simple pulmonary eosinophilia (Loeffler syndrome), chronic eosinophilic pneumonia, and organizing pneumonia. Simple pulmonary eosinophilia is characterized by blood eosinophilia and transient and migratory areas of consolidation that typically clear spontaneously within 1 month. Organizing pneumonia is most frequently manifested as patchy nonsegmental unilateral or bilateral areas of consolidation ( Fig. 2.10 ). The consolidation may involve any lung zone but tends to be most extensive in the peripheral lung regions. Organizing pneumonia may be idiopathic (cryptogenic organizing pneumonia) or secondary to a known cause such as infection, drug reaction, collagen vascular disease, or radiation therapy.

Occasionally, chronic multifocal bilateral consolidation may be due to adenocarcinoma or lymphoma. The consolidation in adenocarcinoma may be focal or multifocal and confluent and is usually associated with air bronchograms ( Fig. 2.11 ). The consolidation results from tumor growth along the alveolar walls combined with secretion of mucin. Occasionally, production of copious amounts of mucin may result in expansion of the lobe and bulging of the interlobar fissures (bulging fissure sign). Pulmonary lymphoma may result in single or multiple mass-like areas of consolidation or, less commonly, extensive confluent areas of consolidation ( Fig. 2.12 ). The areas of consolidation usually contain air bronchograms.
Acute
- •
Pneumonia
- •
Bacteria, mycobacteria, fungi, viruses
- •
Aspiration
- •
- •
Hemorrhage
- •
Secondary to focal disease such as bronchiectasis or carcinoma
- •
Contusion (trauma)
- •
Vasculitis
- •
Infarct (pulmonary thromboembolism)
- •
- •
Pulmonary edema
- •
Reexpansion edema
- •
Papillary muscle dysfunction (right upper lobe edema)
- •
Blood flow redistribution in severe pulmonary thromboembolism
- •
Pulmonary vein occlusion
- •
Chronic
- •
Pneumonia
- •
Eosinophilic pneumonia
- •
Organizing pneumonia
- •
Lipoid pneumonia
- •
Neoplasm
- •
Postobstructive pneumonitis distal to endobronchial carcinoma
- •
Adenocarcinoma, lymphoma
- •


Extensive Confluent and Diffuse Consolidation
A number of conditions may result in extensive or diffuse bilateral consolidation. Because the radiologic appearance is often similar regardless of cause, it is essential to know the clinical history (e.g., trauma, known systemic disease), presence or absence of fever, and immune status of the patient.
Extensive or diffuse bilateral consolidation is seen most commonly in patients with hydrostatic pulmonary edema, permeability edema such as ARDS, diffuse pulmonary hemorrhage, and Pneumocystis pneumonia. In hydrostatic pulmonary edema the consolidation tends to involve mainly the perihilar regions (known as a butterfly or batwing distribution) and is commonly associated with thickening of the interlobular septa (septal lines) and cardiomegaly ( Fig. 2.13 ). Other conditions that may result in a predominantly perihilar distribution include diffuse pulmonary hemorrhage, inhalational lung injury, alveolar proteinosis, and Pneumocystis jirovecii pneumonia ( Fig. 2.14 ). P. jirovecii pneumonia, seen most commonly in patients with AIDS, typically progresses radiographically from a subtle perihilar haze to diffuse bilateral consolidation. Pleural effusions are typically absent.