Fig. 12.1
Contrast-specific CEUS imaging. (a) 21 s after contrast injection. The contrast-only image shows an almost black picture, demonstrating optimal tissue suppression. (b) 25 s after injection. Start inflow of contrast. (c) 45 s after injection. Peak enhancement. (d) 130 s after injection. Slow decrease of contrast concentration. Each panel: left, contrast-only; right, normal grayscale imaging
In 2005, a comparison between contrast-enhanced harmonic imaging targeted biopsies and systematic biopsies was performed by Halpern et al. Biopsies were performed in 301 patients. CEUS-targeted biopsies were two times more likely to find cancer compared to systematic biopsies in patients with PCa. However, targeted biopsies missed 20% of cancers, which were detected by systematic biopsies alone. They concluded that although the detection rate of carcinoma is higher with CEUS-guided biopsies, systematic biopsies are still needed [26].
In 2010, Matsumoto et al. compared radical prostatectomy histology results with preoperatively performed harmonic imaging CEUS and grayscale US in 50 patients. Using grayscale US, they were able to identify at least one tumor focus in 40% of the cases. When using CEUS alone, at least one enhanced tumor focus was seen in 62% of the patients, and when combined, in 80% of the cases identification of a tumor focus was possible [27].
Sano et al. used harmonic imaging to perform targeted biopsies in 41 patients, and compared them with 12-core systematic biopsies. Just like Halpern et al., they also found significantly more cancers using CEUS-targeted biopsies (36.6%) in comparison with systematic biopsies (17.7%). Furthermore, they compared radical prostatectomy tumor locations with preoperatively performed CEUS in 13 patients. In ten patients at least one tumor could be identified [28].
CEUS seems a promising imaging technique in diagnosing PCa. It can be performed at relatively low costs, is repeatable, and has minimal health risk. One of the biggest downsides of CEUS at this time is the interpretation and analysis of the data. The perfusion and contrast-enhanced lesions are subjectively categorized by the investigator, which gives rise to two problems. Clearly two different investigators will interpret the same image differently, but also every investigator has to perform a substantial amount of CEUSs before being able to interpret the images correctly.
Analysis of CEUS is complex; there is only a short window of time to judge the inflow of microbubbles. Furthermore, slight changes in enhancement and timing of events are difficult to detect by the eye. This makes interpretation of CEUS for PCa difficult outside the centers of excellence. A more objective and reliable interpretation by quantification of CEUS is needed to overcome this problem.
Quantification
The first attempts for quantification of CEUS in PCa used perfusion-related parameters. In 2003, the contrast in- and outflow characteristics after a bolus injection were studied by Goossen et al. using Power Doppler. They were able to identify in which side of the prostate the largest malignancy was located in 78% of the cases by analyzing the time between injection and maximum peak of contrast enhancement [29].
Recently, Kuenen et al. presented a quantification method based on the diffusion or dispersion of contrast agent in tissue [30]. They determined on a pixel basis the spreading of contrast in the tissue by using a mathematical model of diffusion. For this model, a diffusion-related parameter is extracted from measured time intensity curves. For an example see Fig. 12.2. A preliminary evaluation in four patients scheduled for radical prostatectomy was performed. In this study, the correlation between the diffusion parameter and the histology was determined. Based on a pixel comparison, the area under the ROC curve was 0.909. This is superior to that of any other measured perfusion-related parameter as proposed in the literature until now. They hypothesize that the intravascular diffusion or dispersion, as described by the extracted diffusion parameter, correlates with the microvascular structure and therefore correlates better with angiogenesis than the traditional perfusion parameters.
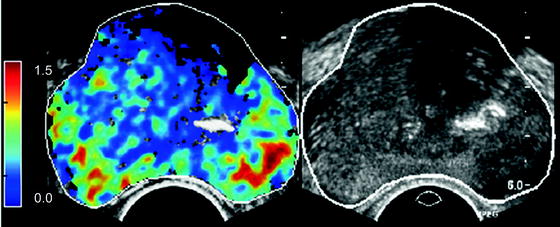

Fig. 12.2
Left, parametric image of dispersion parameter. Blue, low dispersion, red, high dispersion value. Right, grayscale image
Another investigation using hemodynamic parameters in harmonic imaging was performed by Zhu et al. In 103 patients ROIs were drawn in systematic biopsy sites and areas of sonographic abnormalities, e.g., heterogeneous contrast flow, abnormal Doppler flow, echotexture, or contour deformity. A comparison between Gleason score and the arrival time (AT), time to peak (TTP) and peak intensity for these ROI curves was performed. No significant difference was detected between enhancement of low-grade tumors and nonmalignant tissue. However, high-grade tumors had a significantly shorter AT and TTP than low-grade tumors [31].
In conclusion, multiple studies show that quantification can make a reliable and objective interpretation of CEUS possible with a high sensitivity. These promising results have to be further confirmed in larger studies though. More evidence is still needed to judge the role of quantification in a routine clinical environment. At this moment, multiple contrast bolus injections are required because for every bolus of contrast, only 1 single 2D plane can be examined. Quantification techniques using 3D/4D CEUS have not been described yet, but potentially can solve this limitation. Additionally they enable analysis of diffusion and perfusion in 3D, which most probably will further improve the accuracy of the techniques.
Focal Therapy
Low-grade PCas (Gleason 3 or lower) grow slowly and, even if untreated, rarely progress to symptomatic disease. The appeal of watchful waiting strategies is offset by the lack of validated means to identify appropriate candidates, monitor progression, and initiate delayed therapy without compromising chances of cure. On the other hand, surgery and irradiation each provide excellent long-term cancer control, but they are accompanied by a risk of side effects that decrease quality of life. If it concerns a localized lesion, focal therapy may be a fitting option to still treat the cancer but lower the risk of side effects accompanying radical treatment.
Up to today, the numbers of articles on the use of CEUS in focal therapy are still scarce. In 2007 Wondergem et al. reviewed the role of CEUS in the treatment of high-intensity focused ultrasound (HIFU) and cryoablation [32]. They concluded that CEUS can be used as a verification of the used therapy, using the blood flow as an indicator for treatment effects. After successful HIFU or cryoablation treatment, the blood flow reflects the affected tissue and should be absent in the treated area. For an example see Fig. 12.3. Follow-up with CEUS can be used to see if in the treated area blood flow remains absent. At the same time, CEUS imaging of the untreated area is possible to determine if this area remains free of PCa.
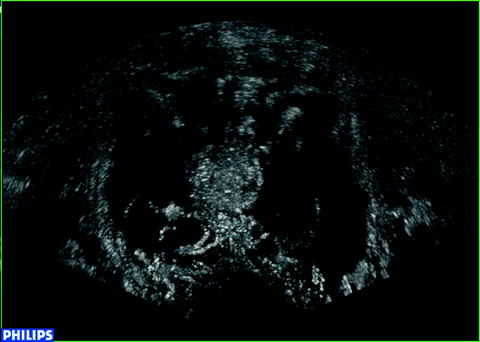

Fig. 12.3
Contrast image after bilateral cryoablation. Ablated areas show less enhancement
Recently, Rouviere et al. compared CEUS imaging with tissue ablation with targeted biopsies after HIFU treatment in 28 patients [33]. They showed that within minutes after the treatment and remaining up till 30–45 days after HIFU ablation, no enhancement is seen in the target area. Peripheral enhancement was present only outside the target areas. After biopsy, viable gland tissue (benign or malignant) was found in 6.2% of all sites with no enhancement on CEUS. In biopsies of mild and/or patchy enhancement, 34% viable tissue was found, and in biopsies of strong/-marked enhancement, 60% viable tissue was found. This makes CEUS a promising method for assessing the extent of ablated tissue after HIFU treatment, and assist in the re-treatment considerations in case of incomplete tissue destruction.
Another possible application of CEUS is real-time monitoring of thermal ablation of localized PCa, as shown by Atri et al. [34]. They described a patient with a single-focus PCa who underwent interstitial laser thermal focal therapy. Before, during, and after the ablation therapy they performed a CEUS. During and after the treatment, large devascuralized zones were seen around the laser-fiber locations. Also increase of the devascuralized zones was observed when more thermal energy was administered. After 1 week, volume measurement of the ablated area was performed using T2– and gadolinium-enhanced MRI. This showed a lesion size similar to the CEUS measurement.
Concluding, CEUS has shown to be a promising tool for real-time monitoring and follow-up of different focal therapy methods. More studies are needed to determine the exact role of CEUS for clinical use in the detection and treatment of PCa.
Molecular Imaging
Targeted microbubbles are a new generation of ultrasound contrast agents. In these bubbles, additional molecules that bind to specific intravascular receptors are embedded in the shell. Possible receptor targets for PCa are those that are upregulated during the process of angiogenesis. Most research has been focusing on the vascular endothelial growth factor (VEGF) receptors.
These targeted microbubbles have the same general features as traditional microbubbles and can be visualized in the same way. However, the bubbles will attach to the target receptors after an intravenous injection, and after some time, the concentration of free floating microbubbles will be significantly lower than the concentration of the attached bubbles in the tissue where the receptors are upregulated. A big advantage of these microbubbles is the larger time window for detecting lesions; after approximately 7–10 min, once the bubbles are bound, the whole organ can be scanned for minutes.
Recently, research using targeted microbubbles has been performed in vitro as well as in vivo [35–38]. Fischer et al. and Tardy et al. investigated the contrast-enhancing effects of target-specific microbubbles versus a nonspecific contrast agent in malignant prostatic tissue in a rat model. Contrast enhancement was still visible in the prostate tumor more than 10 min after infusion of a low concentration of VEGF-R2 targeted contrast agent. Also, the peak intensity and the increase in signal intensity (wash-in rate) of both the nonspecific contrast agent and the targeted contrast agent in malignant tissue were significantly higher than in normal tissue [35, 37]. Although these experiments in animal models showed the effectiveness and potential use of targeted contrast agents in the diagnosis of PCa, most agents use target ligands that cannot be used in humans. Due to foreign protein content in the shell of the microbubble, a potentially immunogenic response can manifest. However, recently Pochon et al. presented a targeted bubble designed for use in humans. They demonstrated an avid receptor bond to cells expressing VEGFR2 using a biospecific lipopeptide in an in vitro human PCa animal model [36]. These results pave the way open the door to clinical research of targeted microbubbles in humans.
In conclusion, promising results of tumor detection using targeted microbubbles has been demonstrated in rat prostate models. Recently, targeted contrast agents designed for clinical use in humans have been reported. Therefore it can be expected that contrast agents targeting human tumor receptors will become available for clinical testing in the near future.
Future
All abovementioned studies in this chapter were performed at academic centers standing at the forefront of contrast-enhanced TRUS developments. Furthermore, only single-institutional studies have yet been performed. Thus, it still has to be determined how these novel techniques will fare in regular clinical centers and in multi-institutional setups. These studies should also answer the question of how steep the learning curve is for radiologists and urologists who are new to the field of contrast-enhanced TRUS.
Focal therapy will keep on growing over time. With this, proper staging is becoming even more important. Even today, when the majority of PCa is treated by removing or irradiating the whole prostate, a recurrence rate of 25% is seen. With the use of targeted contrast, good differentiation between unifocal and multifocal cancer is possible, and a better image of tumor growth into the capsule or seminal vesicles is achieved. Also the detection of higher Gleason score cancers on contrast-enhanced target biopsy may allow for better screening detection of PCa [39]. By using only contrast-enhanced-targeted biopsy, low Gleason score cancers may not be detected, which may decrease overdiagnosis and overtreatment of patients with PCa [40].
Diagnostic requirements before focal therapy include exclusion of cancer in other locations of the prostate. Although at the moment the existing CEUS modalities are not sufficiently reliable, new developments, such as described above, can change this. If CEUS can support the diagnosis and extent of the PCa, and show that other parts of the prostate are free from cancer, there is no more need for extended saturation biopsy schemes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree