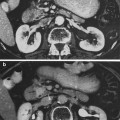
Fig. 1
The biocontinuum of RT-induced heart injury (Published with kind permission of Saunders: Rubin Casarett: Clinical Radiation Pathology 1968)
2 Anatomy and Physiology: The Functional Unit
2.1 Anatomy
The cardiovascular system consists of the heart, arteries, and veins.
The heart is a hollow, muscular organ lying within a two-layered sac, the pericardium. It is a four-chamber pump consisting of two atria and two ventricles. The heart valves maintain the unidirectional flow of blood in the heart by opening and closing depending on the difference in pressure on each side. The valves between the atria and ventricles prevent backflow of blood from the ventricles to the atria during systole. In addition, the semilunar valves between the heart and the aorta and the heart and the pulmonary arteries prevent backflow from the aorta and the pulmonary arteries into the ventricles during diastole. The heart valves do not have a blood supply, but they are covered with a specific type of endothelium.
Although the heart is filled with blood, the heart muscle requires its own blood supply, namely the coronary arteries. There are two main coronary arteries, the left and right. Both of these arteries originate from the root of the aorta, immediately above the aortic valve. The left main coronary artery (or left main trunk) branches into the circumflex artery (LCX) and the left anterior descending artery (LAD), and the right coronary artery (RCA) branches into right marginal artery (RMA) and the posterior descending artery (PDA). The circumflex artery supplies the left atrium, the side, and back of the left ventricle. The left anterior descending artery supplies the front and bottom of the left ventricle and the front of the septum. The right coronary artery supplies the right atrium, the right ventricle and the bottom portion of both ventricles and the back of the septum (see Fig. 2).
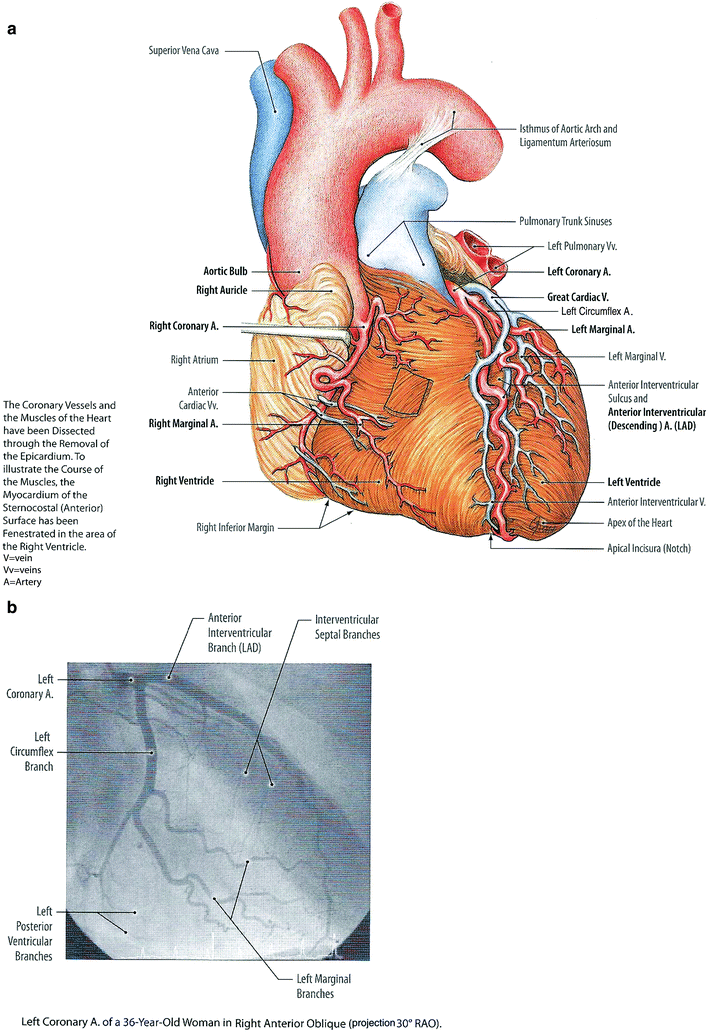

Fig. 2
a Gross anatomy of the heart with an emphasis on depiction of coronary arteries. b Selective coronary angiogram using an anteroposterior projection demonstrating the left coronary arteries and interventricular branches (with permission from Tillman 2007)
2.2 Histology and Functional Subunit
The major part of the heart, the myocardium, is constituted of cardiac muscle. The inner surface of the myocardium is lined with endocardium, and the outer surface with epicardium. The epicardium is the inner visceral layer of the pericardium. The outer part of the epicardium is lined with mesothelium. Large blood vessels and nerves are found in the epicardium.
All arteries possess three coats: a tunica intima, a tunica media, and a tunica adventitia. The tunica intima is composed of a smooth layer of thin endothelial cells based upon a delicate basement membrane that penetrates between the subendothelial connective tissue and the underlying smooth muscle cells. The tunica media consists of smooth muscle cells and an elastic network. The tunica adventitia is a poorly defined layer of connective tissue in which elastic and nerve fibers and in case of large arteries, small, thin-walled nutrient vessels, and the vasa vasorum, are dispersed.
The three separate layers generally seen in arteries are not well defined in veins. Veins are in general thin-walled with relatively large lumina.
The essential function of the heart is to pump blood to various parts of the body. De-oxygenated blood from the body enters the right atrium, passes into the right ventricle and is pumped by the right ventricle to the pulmonary artery into the lungs. Oxygenated blood returns from the lungs to the heart into the left atrium, passes into the left ventricle and is pumped into the body through the aorta. Throughout the body, blood delivers oxygen and nutrients, picks up waste materials, and flows back to the heart again. The muscle wall from the ventricles is thicker and stronger than the muscle wall from the atria.
Cardiac contraction is generated by the myocytes. Myocytes are highly differentiated cells rich in mitochondria. Adjacent myocytes are separated by intercalated disks and they form a network of branching fibers with the ability to carry forward an action potential. Myocytes contract spontaneously and continuously, under regulation of electrical impulses. The electrical impulse initiates in the sinoatrial node (pacemaker), at the junction between right atrium and superior vena cava, and is propagated to the atrioventricular (AV) node, located between the atria and the ventricles. The distal part of the AV node, the bundle of His, splits into two branches to activate the left and right ventricle, respectively. Norepinephrine and its receptors regulate heart rate and the force of contraction.
3 Pathophysiology
3.1 Pathophysiology of Radiation-Related Toxicity
All structures of the heart and major arteries can be damaged by ionizing radiation. See for summary on risk factors Table 1.
Table 1
Risk factors for the different manifestations of radiation-associated heart disease
Risk factor | Pericarditis | Cardiomyopathy | Coronary artery disease | Arrhythmia | Valvular disease | All causes cardiac death | References |
|---|---|---|---|---|---|---|---|
Total dose; (>30–35 Gy) | X | X | X | X | X | X | |
Dose per fraction (≥2.0 Gy/day) | X | X | X | Likely | Likely | X | Cosset et al. (1988) |
Volume of heart exposed | X | X | X | Likely | Likely | X | |
Younger age at exposure Increased | – | X | X | Likely | Likely | X | Aleman et al. (2007) |
Increased time since exposure | – | X | X | X | X | X | Aleman et al. (2007) |
Use of adjuvant cardiotoxic chemotherapy | – | X | – | X | X | X | |
The presence of other known risk factors in each individual such as current age, weight, lipid profile, and habits such as smoking | – | – | X | – | – | X |
3.1.1 Heart Muscle
The normal adult heart is a slow turnover organ, with low proliferative activity. Previously, it was thought that cardiomyocytes were terminally differentiated, without the capacity for cell division. In order for the myocardium to maintain its vital role, it was assumed that loss of myocytes as a result of injury or aging was compensated by hypertrophy of remaining myocytes or by fibrosis. Recent studies have shown that the mammalian heart has the inherent ability to replace its cardiomyocytes through the activation of a pool of resident primitive cells or the recruitment of hematopoietic stem cells (Anversa et al. 2007).
In addition, there is new evidence that circulating mononuclear cells, including progenitor endothelial cells, can home to sites of ischemic damage in the heart and contribute to new vessel formation by transdifferentiation into endothelial cells and secretion of angiogenic cytokines (Caplice and Doyle 2005).
3.1.2 Arteries
Radiation may damage the endothelium of blood vessels. In large arteries this damage may lead to accelerated atherosclerosis and an increased risk of vascular stenosis and thromboembolism (Stewart et al. 1995; Veinot and Edwards 1996; Adams and Lipshultz 2005). Experimental data showed that early inflammatory changes in the endothelial cells of irradiated large vessels may lead to monocyte adhesion and trans-migration into the subendothelial space. In the presence of elevated cholesterol levels, these invading monocytes transform into activated macrophages, which ingest lipids and form fatty streaks in the intima, thereby initiating and accelerating the process of atherosclerosis. Proliferation of myofibroblasts is then stimulated by the production of inflammatory cytokines, resulting in a reduction of the arterial lumen (Vos et al. 1983; Tribble et al. 1999; Stewart et al. 2006). Animal studies have shown that radiation predisposes to the formation of macrophage rich, unstable plaque, rather than stable collagenous plaque (Stewart et al. 2006; Pakala et al. 2003). Such lesions are more likely to rupture and cause a fatal heart attack or stroke (Stewart et al. 2010) (see Fig. 3 for histopathology, and Fig. 4 for pathogenesis of vessel injury).
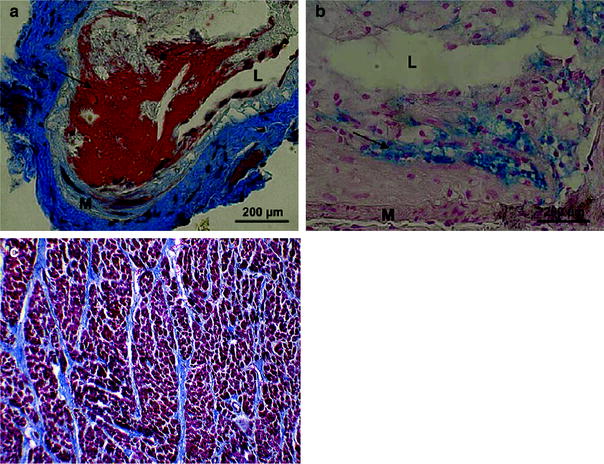
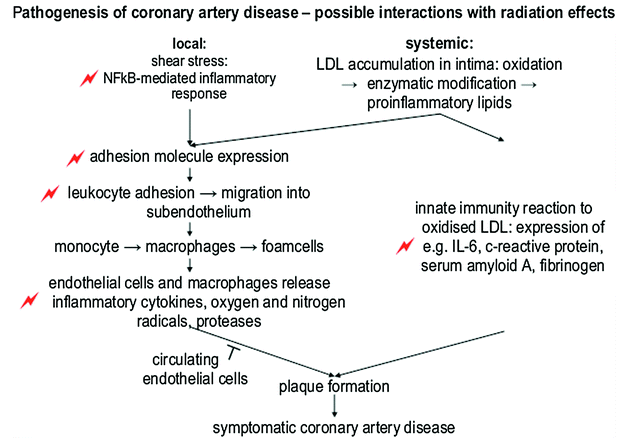

Fig. 3
Pathology. Examples of thrombotic phenotypes of carotid lesions in irradiated ApoE -/- mice. a Martius-scarlet-blue staining of lesions 30 weeks after 14 Gy, in which fibrin deposits are stained red (arrow). b Perl staining in which iron is stained blue (arrow) in lesions 34 weeks after 20 Gy × 2.0 Gy. L = lumen; M = media. (From Hoving et al. 2008). c Example of myocardial fibrosis (fatal) in a patient many years after irradiation for Hodgkin Lymphoma. Whereas normally there should be very little collagen among the dark red myocytes, this heart muscle is criss-crossed by multiple bands of blue collagen. Gomori trichrome stain (with permission from Darby et al. 2010)

Fig. 4
Schematic representation of the most important steps of pathogenesis of coronary artery disease. Events that have also been observed after radiation are indicated by flashes (with permission from Schultz-Hector and Trott 2007)
The anterior location of the heart in the chest, and the relative anterior location of the coronary arteries (in particular the LAD) within the heart, has been suggested to explain the increased risk of radiation-associated heart disease that is observed when thoracic radiation treatment is “weighted” to be preferentially delivered from the anterior direction (Stewart et al. 1995; Byhardt et al. 1975; Morton et al. 1973).
3.1.3 Valves
Since valves do not have blood vessels, radiation-related valvular disease cannot be explained by (micro) vascular damage. However, possibly this damage is consequential to late injury of the surrounding myocardial endothelium leading to fibrosis (Veinot and Edwards 1996).
Valvular leakage could also be secondary to dilated cardiomyopathy. Cardiomyopathy is usually caused by chemotherapy (especially anthracyclines) but may also be seen after radiotherapy probably secondary to vascular damage.
3.1.4 Pericardium
In experimental studies in dogs, rabbits, or rats a single dose of greater than or equal to 15 Gy has been shown to lead to a reversible exudative pericarditis, occurring at around 100 days (Schultz-Hector 1992). Edematous swelling, fibrotic thickening, and adhesions of epicardium and pericardium may develop (Schultz-Hector and Trott 2007; Lauk et al. 1985).
In mammals, the encasement of the heart by a rigid nonpliable pericardium results in characteristic pathophysiologic effects, including impaired diastolic filling of the ventricles, exaggerated ventricular interdependence, and dissociation of intracardiac and intrathoracic pressures during respiration.
In humans, acute pericarditis/pericardial effusion may be observed following irradiation including a considerable part of the heart (Carmel and Kaplan 1976; Cosset et al. 1988). Pericardial effusions may resolve spontaneously. However, constrictive pericarditis does develop in a minority of patients and surgery may be needed (see below).
3.2 Pathophysiology of Chemotherapy-Induced Cardiotoxicity
The mechanisms of cardiotoxicity vary widely among different chemotherapeutics (Kang 2001). The cardiovascular system has numerous different targets that can be subject to damage (Albini et al. 2010). Some drugs directly damage cardiomyocytes or cause inflammation of the pericardium. Some drugs damage the intima of blood vessels and thereby engage the coagulation system, promoting blood clotting in the vessels predisposing to thromboembolic events and consequent cardiovascular and cerebrovascular ischemia. Some drugs cause hypertension with acute and long-term effects on cardiac hypertrophy and insufficiency. See for summary on potential mechanisms and risk factors Tables 2 and 3.
Table 2
Potential mechanisms of cardiovascular damage induced by anticancer treatments. A summary of probable mechanisms of cardiotoxicity induced by a range of chemotherapeutic and chemoprevention agentsa from Albini et al. JNCI 2010 (Albini et al. 2010)
Effects | ||||||||
|---|---|---|---|---|---|---|---|---|
Systemic-macroenvironment | ||||||||
Mitochondrial | Local-microenvironment | |||||||
Examples of chemotherapeutics | Possible cardiovascular damage | DNA damage | ATP block | Apoptotic protein release | ROS generation | Endothelial cell damage/spasms | Cell signaling/survival block | ADCC |
Anthracyclines and anthraquinolones | CHF, LVD, acute myocarditis, and arrhythmia | + | + | + | + | − | − | − |
Capecitabine, 5-fluorouracil, cytarabine | Ischemia, pericarditis, CHF, and cardiogenic shock | + | + | + | + | + | − | − |
Paclitaxel, vinca alkaloids | Sinus bradicardia, ventricular tachycardia, atrioventricular block, hypotension, CHF, and ischemia | + | ? | ? | ? | ? | − | − |
Cyclophosphamide | Neurohumoral activation, mitral regurgitation | + | ? | ? | ? | + | − | − |
Imatinib | Arrythmias, CHF, angioedema, and LVD | − | + | + | − | < > | < > | − |
Sorafenib | Hypertension, arrhythmias | − | ? | ? | − | < > | < > | − |
Sunitinib | Hypertension, arrhythmias | − | + | ? | − | < > | < > | − |
SERMs | LDL/HDL modulation, thromboembolism | − | − | − | − | – | − | − |
Trastuzumab | Arrhythmias, CHF, angioedema, and LVD | − | − | − | − | < > | < > | + |
Bevacizumab | Hypertension, thromboembolism, and GI tract bleeding | − | − | − | − | < > | < > | − |
COX-2–specific inhibitors | Thromboembolism | − | − | − | − | < > | − | − |
Thorax irradiation | Myocardial fibrosis, valvular heart disease, and LVD | + | − | < > | + | + | − | − |
Table 3
Risk factors for Anthracycline-induced cardiotoxicity in decreasing order of importance
Risk factor | Features |
|---|---|
Total cumulative dose | Most significant predictor of abnormal cardiac function |
Age | For comparable cumulative doses, younger age predisposes to greater cardiotoxicity |
Length of follow-up | Longer follow-up results in higher prevalence of myocardial impairment |
Gender | Females more vulnerable than males for comparable doses |
Concomitant mediastinal irradiation | Enhanced toxicity; not clear whether additive or synergistic |
3.2.1 Anthracyclines
Anthracycline-induced cardiotoxicity has been divided into acute, occurring immediately after infusion of the drug, early onset chronic progressive, occurring during or within a year after treatment, and late-onset chronic progressive, occurring more than a year after treatment with anthracycline.
Several different biochemical changes are seen in cellular and animal studies after exposure, and the cardiotoxicity after exposure is likely to be the result of several biochemical insults (Barry et al. 2007; Giantris et al. 1998; Herman et al. 1999; Wouters et al. 2005). Iron-mediated free radical formation leading to apoptosis seems to be important for acute cardiotoxicity (Minotti et al. 2004). However, chronic cardiomyopathy seems to develop due to a combination of diverse processes as follows: increased membrane lipid peroxidation; inhibition of nucleic acid and protein synthesis; release of vasoactive amines; changes in adrenergic function and adenylate cyclase; abnormalities in the handling of Ca2+; reduced expression of specific genes possibly caused by altered expression and function of anthracycline sensitive transcriptional regulatory proteins; impairment of membrane binding, assembly, and enzymatic activity of mitochondrial creatine kinase; induction of nitric oxide synthase, leading to nitric oxide and peroxinitrite formation and to inactivation of myofibrillar creatine kinase or activation of metalloproteinases (Wouters et al. 2005; Minotti et al. 2004). Whether and how these processes contribute to the development of chronic cardiotoxicity is still not clear. Moreover, it is not clear how iron and reactive oxygen species interact with these processes.
Compared with the cells of other organs, cardiac cells are more susceptible to free radical damage because of the highly oxidative metabolism and relatively poor antioxidant defenses (Doroshow et al. 1980). Additionally, anthracyclines have a high affinity for cardiolipin present in the inner mitochondrial membrane leading to accumulation of anthracyclines in cardiomyocytes (Goormaghtigh et al. 1990).
The anthracycline damage to cardiomyocytes causes apoptosis, thereby decreasing the number of myocardial cells (Arola et al. 2000). The wall of the left ventricle becomes thinner, and the contractility of the myocardium decreases, leading to depressed overall function of the left ventricle (Lipshultz et al. 1991; Giantris et al. 1998; Sorensen et al. 1995).
Recent research indicates that anthracyclines cause damage to the cardiac progenitor cells in particular, causing massive apoptotic death, thus reducing the reserve of functionally competent cardiac progenitor cells. This process may contribute to the development of anthracycline-mediated cardiomyopathy in children. In adults, decrease in cardiac stem cells may impair cardiac response to injury. The cumulative effects of anthracyclines on various types of cardiac tissue are summarized in Fig. 5 (Chen et al. 2011). In the future, cardiac progenitor cells may have a potential role in ameliorating cardiotoxicity of cancer therapy. A recent study in rats suggested that it might be possible to prevent cardiomyopathy caused by chemotherapy by obtaining cardiac progenitor cells before initiating cardiotoxic treatment and using them for prevention or management of heart failure (De Angelis et al. 2010).
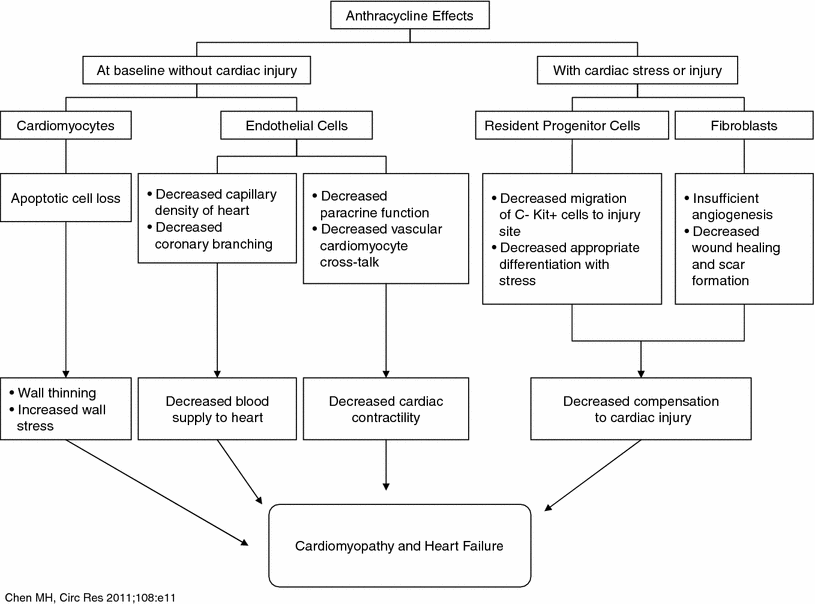

Fig. 5
Anthracycline effects on different cardiac tissues (including myocytes and stems cells) and how it results in heart failure (with permission Chen et al. 2011)
A number of different drugs have cardioprotective properties against anthracycline cardiotoxicity (Wouters et al. 2005). However, due to the risk that these drugs may also reduce the anti-tumor activity of the anthracyclines, they have not gained acceptance except for very special clinical situations.
3.2.2 Alkylating Agents
Cyclophosphamide cardiotoxicity is characterized by hemorrhagic myocarditis in the acute phase. The precise mechanism is unknown, but it is hypothesized that cyclophosphamide causes direct endothelial injury leading to extravasation of toxic metabolites resulting in damage to cardiomyocytes, interstitial hemorrhage, and edema (Goldberg et al. 1986; Gottdiener et al. 1981; Morandi et al. 2005; Pai and Nahata 2000). Microemboli in cardiac capillaries may develop, giving rise to ischemic myocardial damage (Gottdiener et al. 1981; Morandi et al. 2005). Coronary vasospasm is another possible mechanism (Pai and Nahata 2000).
Ifosfamide cardiotoxicity may possibly cause myocardial damage by the same mechanisms as cyclophosphamide, but hemorrhagic myocarditis has not been described, so other unknown mechanisms may be at play (Quezado et al. 1993).
3.2.3 Antimetabolites
Fluorouracil and its prodrug capecitabine may cause cardiotoxicity by mechanisms which are as yet not fully elucidated. Coronary vasospasms have been proposed (Akhtar et al. 1993; de Forni et al. 1992; Kosmas et al. 2008; Luwaert et al. 1991; Shoemaker and Arora 2004; Sudhoff et al. 2004; Wacker et al. 2003). However, there is also evidence for a direct effect on the metabolism of the cardiomyocytes (de Forni et al. 1992; Kohne et al. 1998; Sasson et al. 1994). Symptoms usually start several hours after repeated administrations of the drug. In view of the short half-life of fluorouracil, it seems likely that cardiotoxicity is caused by metabolites which accumulate. Evidence points to metabolites formed by dihydro-pyrimidine dehydrogenase degradation of fluorouracil (Di Paolo et al. 2001; Van Kuilenburg et al. 2002).
3.2.4 Antimicrotubule Agents
Myocardial ischemia and infarction have been associated with paclitaxel and docetaxel administration. The etiology is thought to be multifactorial, including histamine release by the cremophor of the paclitaxel formulation (Rowinsky et al. 1991). Paclitaxel may also cause arrhythmias, possibly via direct effects on the Purkinje system or extracardiac autonomic control (McGuire et al. 1989), but also via histamine release by the cremophor (Rowinsky et al. 1991; Arbuck et al. 1993).
Vinca alkaloids can also cause arrhythmias, ischemia, and congestive heart failure (Yeh et al. 2004).
3.2.5 Monoclonal Antibodies
Monoclonal antibodies used in cancer therapy typically target growth factors or their receptors thereby inhibiting the tyrosine kinases normally activated through interaction between the growth factor and the corresponding receptor.
3.2.5.1 Bevacizumab
Arterial thrombotic events, including myocardial infarction, are associated with treatment with bevacizumab. The mechanism is unclear, but it is thought that anti-vascular endothelial growth factor (VEGF) therapy may decrease the capability of endothelial cells to regenerate in response to trauma, leading to defects in the interior vascular lining with exposure of subendothelial collagen, increasing the risk of thrombotic events (Kamba and McDonald 2007; Kilickap et al. 2003).
Furthermore, antiangiogenic therapy is known to cause hypertension by mechanisms which are not fully understood. It is possibly related to VEGF inhibition, which decreases nitric oxide production in the arterioles (Kamba and McDonald 2007). Nitric oxide is a natural vasodilator, and blocking its production leads to increased peripheral vascular resistance and blood pressure. Angiogenesis is an important component of the normal adaptive response to pressure load, and inhibition of VEGF signaling may be even more serious in hypertensive patients (Izumiya et al. 2006; Shiojima et al. 2005).
3.2.5.2 Trastuzumab
Human epidermal growth factor receptor 2 (HER2) has an essential role in the development of the embryonic heart (Erickson et al. 1997; Lee et al. 1995a), and HER2 is expressed on adult myocardiocytes (Strasser et al. 2001; Zhao et al. 1998). Preclinical experiments have shown that activation of the HER2 receptor induced by its ligand neuregulin promotes cardiomyocyte survival (Zhao et al. 1998). Cardiotoxicity of trastuzumab is most likely secondary to inhibition of HER2 receptor on cardiomyocytes, thereby interfering with their normal growth and repair, whereas there is little evidence of cell death in the adult myocardium due to HER2 inhibition, which may explain the reversibility of trastuzumab cardiotoxicity (Crone et al. 2002; Ewer and O’Shaughnessy 2007; Ozcelik et al. 2002; Suter et al. 2007). However, other mechanisms not involving HER2 signaling may also be operating (Guglin et al. 2008).
3.2.5.3 Cetuximab and Panitumumab
These antibodies target the epidermal growth factor receptor (EGFR), and no cardiotoxicity has been reported with these two drugs.
3.2.6 Small Molecule Tyrosine Kinase Inhibitors
Cardiotoxicity of tyrosine kinase inhibitors falls into two categories (Chen et al. 2008). The first relates to the tyrosine kinase target for cancer therapy having an important role in normal cardiomyocyte survival. Inhibition therefore causes myocardial dysfunction. The second is characterized by inhibition of a kinase which is not the intended target of the drug but which is important in normal cardiomyocytes. This situation is more likely the broader the range of targets for the tyrosine kinase inhibitor in question.
With the growing number of tyrosine kinase inhibitors being developed and approved, the likelihood will increase that some of them inhibit novel kinase targets for which little clinical data exist on associations with cardiotoxicity (Chen et al. 2008; Chen 2009; Cheng and Force 2010).
3.2.6.1 Lapatinib
Lapatinib cardiotoxicity is likely related to HER2 inhibition, as mentioned previously for trastuzumab. However, the risk associated with lapatinib is smaller. The difference may be due to the fact that monoclonal antibodies cause antibody dependent cellular and complement-dependent cytoxicity which could augment cardiotoxicity (Imai and Takaoka 2006). Moreover, differential inhibition and/or activation by lapatinib compared to trastuzumab of downstream signaling pathways may also be responsible (Spector et al. 2007).
3.2.6.2 Imatinib
Animal studies have shown cardiotoxicity in cardiomyocytes. Cardiotoxicity is most likely due to Abl inhibition in cardiomyocytes (Force et al. 2007; Kerkela et al. 2006). This seems to lead to induction of endoplasmic reticulum stress response, although the mechanisms are not clear. The endoplasmic reticulum stress response leads to cellular apoptosis. Imatinib-induced cardiotoxicity in patients is still an issue of debate (Breccia 2011).
3.2.6.3 Dasatinib
Like imatinib, dasatinib inhibits Abl, and the mechanism of cardiotoxicity may be similar. However, dasatinib inhibits a number of other kinases, which may be involved in the cardiotoxicity (Chen et al. 2008).
3.2.6.4 Nilotinib
Nilotinib, like imatinib and dasatinib, inhibits Abl. However, it seems to have a favorable toxicity profile, and the only cardiotoxicity reported is QT prolongation.
3.2.6.5 Sunitinib
Sunitinib is a multi-target tyrosine kinase inhibitor. It has been shown in human and animal studies to cause mitochondrial damage in cardiomyocytes (Chu et al. 2007) (see Fig. 6), with ensuing heart failure and cardiomyopathy in a minority of patients. These symptoms improve with discontinuation of the agent and are responsive to heart failure therapy. Animal studies suggest that sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase (Kerkela et al. 2009). Further work has also suggested that sunitinib cardiotoxicity is caused by inhibition of ribosomal kinase leading to activation of the intrinsic apoptotic pathway (Force et al. 2007). Hypertension may also play a role (Chu et al. 2007; Khakoo et al. 2008).
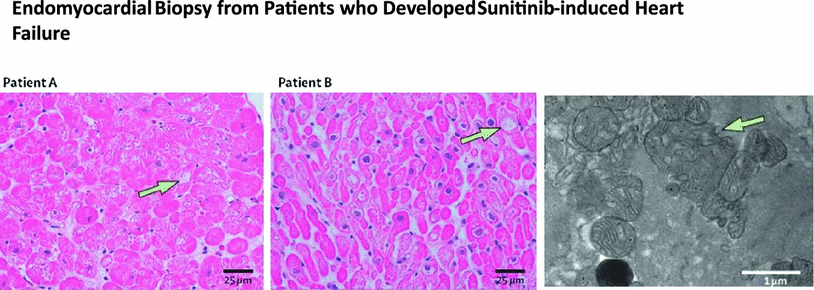

Fig. 6
Endomyocardial biopsy from patients who developed sunitinib-induced heart failure. Representative light photomicrographs from patients A and B (left+middle panels) showed cardiomyocyte hypertrophy with mild degenerative changes and diffuse, and moderate myocyte vacuolization (arrows). There was no edema, interstitial or replacement fibrosis, regional infarct or focal cell necrosis, myocarditis, or inflammation. Transmission election micrograph from patient A (right) showed swollen, abnormal mitochondrial configurations (arrow) with effaced cristae (with permission from Chu et al. 2007)
3.2.6.6 Sorafenib
Sorafenib is also a multi-target tyrosine kinase inhibitor, and it may cause cardiac damage by the same mechanisms as sunitinib. However, sorafenib also causes inhibition of the Raf kinases which may lead to enhanced cardiomyocyte apoptosis and fibrosis of the heart (Yamaguchi et al. 2004).
3.2.6.7 Gefitinib and erlotinib
These drugs target the EGFR tyrosine kinase, and no cardiotoxicity has been reported with these two drugs.
3.2.7 Proteasome Inhibitors
Bortezomib is associated with the development of heart failure, but the mechanism is unknown. It has been proposed that the inhibition of proteasomes in the myocytes is the direct cause of cardiotoxicity (Voortman and Giaccone 2006). In patients with chronic (even subclinical) cardiomyopathy, the ubiquitin–proteasome system is activated, which may be an adaptive mechanism for maintaining a normal stroke volume. In this situation, proteasome inhibition may cause manifest heart failure.
3.2.8 Angiogenesis Inhibitors
With regard to monoclonal antibodies and tyrosine kinase inhibitors acting via the VEGF pathway (bevacizumab, sunitinib), see above.
3.2.8.1 Thalidomide
Thalidomide is associated with the development of bradycardia. The mechanism remains unclear. It has been suggested to be due to the central sedative effects of the drug or to vasovagal activation. Thalidomide reduces the level of TNF-alpha which causes inhibition of the dorsal motor neurons including the nucleus of the vagus nerve. This could conceivably lead to over-reactivity of the parasympathetic nervous system leading to bradycardia. In some patients, thalidomide may cause hypothyroidism which may also lead to bradycardia (Fahdi et al. 2004; Kaur et al. 2003).
3.2.9 Histone Deacetylase Inhibitors
Vorinostat has been associated with QT prolongation. The mechanism is unknown.
3.2.10 Arsenic Trioxide
Arsenic trioxide has been associated with QT prolongation, and the mechanism is unknown.
4 Clinical Syndromes: Radiation Versus Chemotherapy and Interactions
The heart is a complex organ composed of distinct anatomic components that work in synchrony for effective global function. Injury to any one component of the organ can compromise function or render the entire organ dysfunctional. Conversely, the heart also has tremendous reserve, and can sustain a moderate degree of injury that remains subclinical. We will discuss the effects of radiotherapy and systemic therapy on several anatomical parts of the cardiovascular system.
4.1 The Heart
4.1.1 Effects of Radiation on the Heart
4.1.1.1 Radiation-Associated Heart Disease: Epidemiological Data
Radiation-associated heart disease includes a wide spectrum of cardiac disease, and combined disease of the coronary arteries, the heart valves, the myocardium, and the conductive system may occur (Adams et al. 2003; Aleman et al. 2007). These conditions usually only become symptomatic 10–15 years after exposure of the heart to irradiation, leading to an increased risk of (fatal) cardiovascular events after for instance mediastinal irradiation for Hodgkin lymphoma and after irradiation for left-sided breast cancer; symptomatic abnormalities may develop much earlier. (Hancock et al. 1993a; Stewart et al. 1995; Adams et al. 2004; Boivin et al. 1992; Carlson et al. 1991; Clarke et al. 2005; Darby et al. 2003, 2005; Giordano et al. 2005; Glanzmann et al. 1998; Gustavsson et al. 1990; Hojris et al. 1999; Lee et al. 2000; Lipshultz and Sallan 1993; Lund et al. 1996; Paszat et al. 1998; Piovaccari et al. 1995; Rutqvist and Johansson 1990; Swerdlow et al. 2007; Taylor et al. 2006). The reported incidence of injury is thus clearly related to the endpoints being considered. It is often useful to stratify the various endpoints (albeit somewhat imperfectly) into categories as shown in Table 4.
Table 4
Endpoints related to radiation-induced heart disease
Focal | Global | |
|---|---|---|
Subclinical (usually objective) | Abnormalities in regional perfusion or wall motion via SPECT/MRI Valvular abnormalities via ECHO Asymptomatic coronary lesions Pericardial thickening on imaging or a pericardial rub heard on exam EKG abnormalities | Global imaging abnormality (e.g. diffuse hypocontractility) Asymptomatic reduced EF |
Clinical (usually subjective) | Coronary artery disease Myocardial infarction Valvular disease | Congestive heart failure Pericarditis/pericardial effusion Arrhythmia Autonomic dysfunction |
The long delay before expression of serious damage probably explains why the radiation sensitivity of the heart has previously been underestimated.
Information on mortality from cardiovascular diseases (CVDs) has been available in many countries for quite some time, whereas the information of incidence rates of CVD has been scarce. Gradually, incidence rates of several CVDs have become available. In addition, incidence rates of hospitalization for ischemic heart disease (Reinders et al. 1999) and of utilization of valve surgery, percutaneous interventions, and coronary bypass graft surgery among patients with HL (Hull et al. 2003) compared with general population rates, have been used as surrogate markers for CVD incidence.
Epidemiological studies on survivors of Hodgkin lymphoma show relative risk estimates for cardiac deaths in the range of 2–7, depending on the age of the patients (increased risks for irradiation at young age), the radiation therapy methods used, and the follow-up time (Hancock et al. 1993a; Adams et al. 2003; Boivin et al. 1992; Swerdlow et al. 2007; Aleman et al. 2003). In a Dutch study 3- to 5-fold increased standardized incidence ratios (SIR) of various heart diseases were observed in patients treated for Hodgkin lymphoma before the age of 41 years relative to the general population, even after a follow-up of more than 20 years (Aleman et al. 2007). The 25-year cumulative incidence of heart failure or cardiomyopathy with death from any cause as competing risk following mediastinal radiotherapy only was 6.8 %. The persistence of increased risk over prolonged follow-up time is of concern because this implies increasing absolute excess risks over time, due to the rising incidence of cardiovascular diseases with age.
Increased morbidity from cardiac diseases has been widely reported after treatment for breast cancer, especially using older radiotherapy techniques (Adams et al. 2003; Verheij et al. 1994; Gaya and Ashford 2005; Senkus-Konefka and Jassem 2007). The early breast cancer trialists’collaborative group (EBCTCG) evaluated the effects of local treatment on death from breast cancer and other causes in a collaborative meta analysis evaluating 42,000 women. This study showed a clear benefit of radiotherapy for local control and risk of death from breast cancer. However, there was, at least with some of the older radiotherapy regimens, a significant excess of nonbreast-cancer mortality in irradiated women [rate ratio 1.12; standard error (SE) 0.04] mainly from heart disease (rate ratio 1.27; SE 0.07) (Clarke et al. 2005).
The SEER database (surveillance, epidemiology, and end-results cancer registries) analysis also provides evidence of increased risk of myocardial infarction due to radiotherapy (Darby et al. 2005; Paszat et al. 1998). In a cohort of 308,861 women treated for early breast cancer, tumor laterality had no influence on subsequent mortality for women who did not receive radiotherapy. However, for women irradiated in the period of 1973–1982, there was a significant increase in cardiac mortality for left versus right-sided tumor (1.2 at <10 years, 1.42 at 10–14 years and 1.58 at >15 years). For women irradiated between 1983 and 1992, these risks had decreased to 1.04 at <10 years and 1.27 at >10 years.
Another large study (n > 4,000) investigated treatment-specific incidence of cardiovascular diseases in 10-year survivors of breast cancer treated from 1970 to 1986 in the Netherlands (Hooning et al. 2007). When comparing breast cancer patients who did or did not receive radiotherapy, radiation to the internal mammary chain was associated with significantly increased risk of cardiovascular disease (estimated mean, fractionated dose to the heart 6–15 Gy), while for breast irradiation alone no increased risk was observed (estimated mean, fractionated dose to the heart <7 Gy). For patients treated before 1979, radiation was associated with hazard ratios (HR) of 2.6 and 1.7 for myocardial infarct and congestive heart failure, respectively. For patients irradiated after 1979, the risk of myocardial infarct declined toward unity but the risks for congestive heart failure and valvular dysfunction remained significantly increased (HR 2.7 and 3.2, respectively). Smoking and radiotherapy together were associated with a more than additive effect on risk of myocardial infarction (HR = 3.04).
In concert, these studies suggest that radiotherapy for left-sided breast cancer has the potential to increase the risk of cardiac morbidity and mortality, but that these risks are markedly reduced with newer radiation therapy techniques. However, since radiotherapy-induced heart disease is generally believed to not be clinically manifest until >10–15 years post radiotherapy, and since the follow-up duration is shorter in the studies using more modern techniques (vs. the older techniques), additional follow-up is needed to be confident that the more modern techniques are indeed “safe”. The interaction between the duration of follow-up, the ‘era of the radiotherapy’ (taken as a surrogate for the ‘modern-ness’ of the radiotherapy techniques), and the RR of a cardiac event associated with radiotherapy, is shown in Fig. 7. Nevertheless, we are reassured that the more modern approaches markedly reduce the volume of heart exposed to radiotherapy, and that the available data suggest modern radiotherapy for breast cancer is not associated with a marked increase in cardiac events (albeit with modest follow-up time).
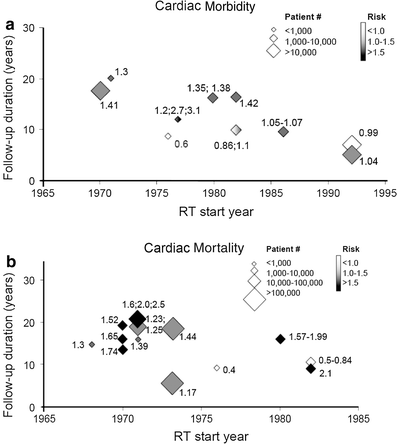
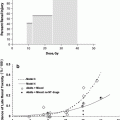
Fig. 7
The interaction of treatment era (based on patient accrual start-year) and follow-up duration on cardiac morbidity (panel a) and mortality (panel b) risk. The number (s) beside each point is the reported ‘risk (s)’ of cardiac morbidity/mortality in the published trial. The size of the diamonds represents trial sample size, and the shading represents the risk of cardiac morbidity (black: >1.5, gray: 1–1.5, and white as ≤1) From Demirci IJROBP 2009
Many factors appear to influence the risk of CVDs. Table 1 summarizes the association between various risk factors and the different cardiac events. The highest relative risks are reported for those treated at young age (Aleman et al. 2007; Mulrooney et al. 2009; Hooning et al. 2007; Chen et al. 2008; Swerdlow et al. 2007; Myrehaug et al. 2008) and only slightly increased risks or no increased risks are reported in patients treated at 65 years or older (Swerdlow et al. 2007).
4.1.1.2 Radiation-Associated Heart Disease: Damage to Coronary Arteries
Although radiation damage to the coronary arteries may be immediate, clinical manifestation of damage does not usually appear until 10 or more years after radiation exposure.
Radiation may cause damage to the vascular endothelium of large arteries and therefore lead to accelerated atherosclerosis and an increased risk of vascular stenosis and thromboembolism.
Because of reduction of the arterial lumen to variable degrees, a spectrum of clinical manifestations of ischemic heart disease may be observed such as stable angina pectoris, unstable angina, myocardial infarction, and chronic ischemic heart disease.
An American study including 961 stage I-II breast cancer patients treated from 1977 to 1995 treated with conventional tangential beam radiation treatment (RT) showed that a statistically significant higher prevalence of stress test abnormalities was found among left (27 of 46; 59 %) versus right-side irradiated patients (3 of 36; 8 %; P < 0.001) at a median follow-up of 12 years. Furthermore, 19 of 27 of left-sided abnormalities were in the left anterior descending artery territory. Thirteen left-side irradiated patients also underwent cardiac catheterization revealing 12 of 13 with coronary stenoses (92 %) and 8 of 13 with coronary stenoses (62 %) solely in the left anterior descending artery (Correa et al. 2007; Tsibiribi et al. 2006a, b; Shapiro et al. 1998).
4.1.1.3 Radiation-Associated Heart Disease: Damage to the Valves
Increased risks of valvular problems (valvular regurgitation in the aortic or mitral valve, and sometimes aortic stenosis) with or without clinical symptoms have been reported following radiotherapy for Hodgkin lymphoma (Aleman et al. 2007; Chen et al. 2011; Adams et al. 2004; Glanzmann et al. 1998; Lund et al. 1996; Heidenreich et al. 2003; Jones et al. 2007).
There are conflicting data following treatment for breast cancer (Hooning et al. 2007; Harris et al. 2006). Progressive valvular dysfunction has also been shown during long-term follow-up after treatment for Hodgkin lymphoma with mediastinal radiotherapy and/or anthracyclines (Wethal et al. 2009). Hodgkin lymphoma patients also have a significantly higher risk (SIR 8.4) of requiring valve surgery 15–20 years after radiotherapy (Hull et al. 2003).
4.1.1.4 Radiation-Associated Heart Disease: Damage to the Pericardium
Acute pericarditis is nowadays uncommon because of improved radiation techniques and lower radiation doses to smaller cardiac volumes. Patients may present with pleuritic chest pain, fever, tachycardia, a pericardial rub, and characteristic electrocardiographic abnormalities. These symptoms and pericardial effusions may resolve spontaneously. Symptomatic treatment analgesic and anti-inflammatory drugs are usually applied to relieve the pain. Constrictive pericarditis does however develop in a minority of patients. Medical treatment may temporarily alleviate symptoms of heart failure, but patients may need a pericardiectomy (Cosset et al. 1991; Bertog et al. 2004; Galper et al. 2010).
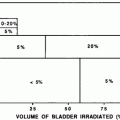
In the past, when generally larger radiation fields were used, for instance, in Hodgkin lymphoma treatment pericarditis was seen relatively frequently. In the early Stanford study, the risk of (a) symptomatic pericarditis was 20 % following whole-heart irradiation to 30 Gy, versus 7 % with the placement of a left-ventricular block at 15 Gy, and 2.5 % with a subcarinal block at 25–35 Gy (Carmel and Kaplan 1976).
Another European study including patients treated several decades ago has estimated that 4.8 % of Hodgkin lymphoma patients treated enveloped pericarditis approximately 18 years post-radiation therapy; the cumulative incidence for pericardial disease requiring surgery in these patients rose from only 0.1 % at 5 years post- radiation therapy to 1.3 % at 25 years from treatment (Galper et al. 2010).
More recently, a variety of dose-volume-histogram-based parameters were reported to be related to pericardial effusion for patients treated for esophageal cancer (Wei et al. 2008; Martel et al. 1998). Martel et al. (1998) implicated fraction size, mean, and maximum dose as a predictors for pericarditis. Wei et al. (2008) reported that a variety of DVH-based parameters (e.g., V3 to V50 and mean dose) predicted for pericardial effusions. The dosimetric parameters were highly correlated with each other, making comparisons of their predictive abilities difficult. See for detailed information Table 5.
Table 5
Pericarditis/pericardial effusion: Dose-volume predictors and NTCP parameters
Authors, year, reference | Diagnosis, No. of patients, years of treatment | OAR | Fractionation schedule, dose data | Predictive parameters | NTCP parameters |
|---|---|---|---|---|---|
Carmel and Kaplana (1976) | Hodgkin lymphoma 377 Patients 1964–1972 | Pericardium | At Dpericardium >30 Gy: 50 % rate of pericarditis, 36 % requiring treatment | ||
Cosset et al. (1991) | Hodgkin lymphoma 499 Patients 1971–1984 | 35–43 Gy/2.5–3.3 Gy/fraction pre-3D dose data | DMediastinum ≥41 Gy, d/fraction ≥ 3 Gy (marginal significance) | ||
Burman et al. (1991) | Historical data | LKBb TD50 = 48 Gy, m = 0.10 n = 0.35 | |||
Martel et al. (1998) | Esophagus 57 Patients 1985–1991 | Pericardium | 37.5–49 Gy/1.5–3.5 Gy/fraction 3D data | Dmean > 27.1 Gyc, Dmax > 47 Gyc, d/fraction 3.5 Gy | LKB (95 % CI) TD50 = 50.6 Gy (−9; 23.1), m = 0.13 (−0.07; 0.13), n = 0.64 (−0.58; 3) |
Wei et al. (2008) | Esophagus 101 Patients 2000–2003 | Pericardium | 45–50.4 Gy/1.8–2.0 Gy/fraction 3D data | Dmean pericardium > 26.1 Gy, V30 < 46 %d |
4.1.2 Effects of chemotherapy on the heart
Several systemic cancer therapies have been associated with the development of left ventricular dysfunction and heart failure.
4.1.2.1 Anthracyclines
Anthracyclines are used to treat a wide range of cancers including breast, uterine, ovarian and lung cancers, leukemias, and lymphomas. The incidence of anthracycline cardiotoxicity depends on the medication and the cumulative dose. For doxorubicin the reported incidence of heart failure is 3–5 % with doses of 400 mg/m2, 7–26 % with doses of 550 mg/m2, and 18–48 % with doses of 700 mg/m2 (Wouters et al. 2005; Swain et al. 1997; Von Hoff et al. 1979). A 5 % risk of cardiomyopathy is seen with a cumulative dose of doxorubicin of 450 mg/m2, of daunorubicin of 900 mg/m2, of epirubicin of 935 mg/m2, and of idarubicin of 223 mg/m2 (Wouters et al. 2005; Keefe 2001), and these doses are generally regarded as the maximum lifetime cumulative dose allowed. However, no threshold dose exists below which no left ventricular dysfunction is seen. Liposomal doxorubicin, epirubicin, and idarubicin seem to have a lower incidence of heart failure. However, the longer the follow-up, the higher the incidence of cardiac dysfunction is reported to be. A higher risk is reported with intravenous high single doses, drug infusion lasting <30 min, prior radiotherapy involving the heart, use of other concomitant agents such as cyclophosphamide, trastuzumab or paclitaxel, female gender, young and old age, and underlying cardiovascular disease (Aleman et al. 2007; Myrehaug et al. 2008; Leonard et al. 2009; Lipshultz et al. 1995, 2008; Moser et al. 2006; Stickeler et al. 2009; Trudeau et al. 2009).
Acute anthracycline cardiotoxicity occurs in <1 % of patients, and manifests immediately after infusion as a reversible depression of myocardial function (Giantris et al. 1998; Wouters et al. 2005). Discontinuation of the anthracycline often leads to improvement in the cardiac function.
Early onset chronic progressive anthracycline cardiotoxicity, usually presenting within 1 year of anthracycline treatment, occurs in 1–3 % of patients. Late onset chronic anthracycline cardiotoxicity occurs at least 1 year after anthracycline treatment in 1.6–5 %, and even higher incidence figures are reported for children, depending on what severity of symptoms are required for the diagnosis. However, it may not become clinically evident until 10–20 or even 30 years after treatment. Chronic progressive cardiotoxicity typically presents as dilated cardiomyopathy (Lipshultz et al. 2008).
4.1.2.2 Alkylating Agents
Cyclophosphamide is an alkylating agent commonly used in the treatment for a variety of nonmalignant and malignant conditions including breast cancer, leukemia, lymphoma, multiple myeloma, mycosis fungoides, neuroblastoma, ovarian cancer, and retinoblastoma (Floyd et al. 2005). Cyclophosphamide has been associated with heart failure in 7–28 % of patients (Goldberg et al. 1986; Gottdiener et al. 1981; Pai and Nahata 2000; Braverman et al. 1991). The risk is dose related, occurring mainly in patients receiving >150 mg/kg and >1.5 g/m2/day. It occurs within 1–10 days after the administration (Pai and Nahata 2000), and the clinical manifestations range from asymptomatic pericardial effusions to overt heart failure and myopericarditis (Gottdiener et al. 1981; Braverman et al. 1991). Cardiac failure following cyclophosphamide usually resolves over 3–4 weeks and is treated with supportive care (Floyd et al. 2005). Risk factors include previous anthracycline treatment and mediastinal radiotherapy (Goldberg et al. 1986; Pai and Nahata 2000).
4.1.2.3 Antimetabolites
Fluorouracil is mainly used in the treatment for patients with cancer of the digestive tract especially colorectal cancer and head and neck cancer. Fluorouracil has been associated with symptoms of cardiotoxicity in 1–18 % of patients, most commonly as angina-like chest pain, but ischemic ECG changes may be seen in up to 68 % (Yeh and Bickford 2009; de Forni et al. 1992; Wacker et al. 2003; Jensen and Sorensen 2006; Keefe et al. 1993; Labianca et al. 1982; Tsavaris et al. 2002). In rare cases myocardial infarction, arrhythmias, heart failure, cardiogenic shock, and sudden death have been reported (Meyer et al. 1997; Van Cutsem et al. 2002). The overall mortality of symptomatic fluorouracil cardiotoxicity has been estimated at 2.2–13 % (de Forni et al. 1992; Wacker et al. 2003; Keefe et al. 1993; Labianca et al. 1982; Tsavaris et al. 2002; Robben et al. 1993). Cardiac events generally occur within 5 days after administration. The risk of cardiotoxicity is associated with high doses (>800 mg/m2), continuous infusion, prior cardiovascular disease or mediastinal radiotherapy, and concurrent chemotherapy (de Forni et al. 1992; Kosmas et al. 2008; Jensen and Sorensen 2006; Labianca et al. 1982; Tsavaris et al. 2002; Meyer et al. 1997; Van Cutsem et al. 2002; Cardinale et al. 2006a, b; Rezkalla et al. 1989).
Capecitabine, an oral prodrug of fluorouracil, has been associated with cardiotoxicity with an incidence of 3–9 % (Kosmas et al. 2008; Van Cutsem et al. 2002; Ng et al. 2005; Saif et al. 2008; Walko and Lindley 2005). Typically, angina symptoms occurred 4–5 days after therapy (Jensen and Sorensen 2006) with ischemic ECG changes present in many cases (Cardinale et al. 2006a; Frickhofen et al. 2002; Schnetzler et al. 2001), but without echocardiography or coronary angiogram abnormalities (Yeh and Bickford 2009; Cardinale et al. 2006a; Schnetzler et al. 2001). Prior fluorouracil cardiotoxicity and possibly prior symptoms of coronary artery disease were risk factors (Labianca et al. 1982; Saif et al. 2008; Frickhofen et al. 2002; Schober et al. 1993).
4.1.2.4 Antimicrotubule Agents
Docetaxel is employed in the treatment of breast cancer and prostate cancer. It has been reported to be associated with heart failure in 2.3–8 % of patients (Martin et al. 2005; Marty et al. 2005). Myocardial ischemia has been reported to occur in 1.7 % of patients (Yeh and Bickford 2009; Vermorken et al. 2007).
Paclitaxel is used in patients with lung cancer, ovarian cancer, and breast cancer. It has been reported to be associated with ischemia in 5 % of patients (Rowinsky et al. 1991) and with myocardial infarction in 0.5 % (Arbuck et al. 1993). The cardiac events occurred up to 14 days after paclitaxel administration (Arbuck et al. 1993), and risk factors were previous cardiac disease including hypertension and coronary artery disease. Paclitaxel has also been associated with bradycardia, which is usually without clinical significance, but may in rare cases have clinically significant hemodynamic effects. Hypersensitivity reactions with histamine release have been implicated, and premedication to prevent hypersensitivity reactions may prevent bradycardia as well.
Vinca alkaloids, used in the treatment of lymphomas, nonsmall cell lung cancer, breast cancer, and testicular cancer, have also been incriminated. Significantly (2 fold or more) increased risks of death from myocardial infarction (Swerdlow et al. 2007) or more general cardiovascular diseases (Tukenova et al. 2010) have been reported.
4.1.2.5 Monoclonal Antibodies
Bevacizumab is a monoclonal antibody that inhibits the activity of human VEGF. It is approved for use in the setting of first-line metastatic colon cancer in combination with intravenous FU-based chemotherapy. It is also used in the treatment of nonsmall cell lung cancer and brain tumors. Bevacizumab has been associated with heart failure in 1.7–3 % of patients (Miller et al. 2005, 2007). Moreover, arterial thrombotic events have been reported in 3.8 % of patients with myocardial infarctions in 0.6 % (Scappaticci et al. 2007). Arterial thrombotic events could occur at any time during therapy, and they did not seem to be related to dose or cumulative exposure. Risk factors were older age and prior arterial thrombotic events (Scappaticci et al. 2007). The most common cardiotoxicity with bevacizumab treatment is, however, hypertension, which is reported to occur in 4–35 % of patients (Miller et al. 2005, 2007; Cobleigh et al. 2003; Hurwitz et al. 2004; Johnson et al. 2004; Kabbinavar et al. 2005; Pande et al. 2007; Yang et al. 2003). Hypertension might develop at any time during therapy, and higher dose has been suggested as a risk factor (Kabbinavar et al. 2005). Most patients can continue bevacizumab in combination with antihypertensive drugs.
Trastuzumab, used in patients with breast cancer, is associated with cardiomyopathy in 2–7 % of patients when the drug is used alone. However, when trastuzumab is used with paclitaxel the incidence is 2–13 %, and when used with an anthracycline it is up to 27 % (Ewer and O’Shaughnessy 2007; Suter et al. 2007; Gianni et al. 2007; Guarneri et al. 2006; Perez et al. 2008a; Romond et al. 2005; Seidman et al. 2002; Slamon et al. 2001; Tripathy et al. 2004; Vogel et al. 2002). Risk factors are age >50, pretreatment borderline left ventricular ejection fraction, prior cardiovascular disease, and prior treatment with anthracyclines to higher cumulative doses (287 mg/m2 vs. 257 mg/m2 for doxorubicin, 480 mg/m2 vs. 422 mg/m2 for epirubicin) (Suter et al. 2007; Guglin et al. 2008; Gianni et al. 2007; Guarneri et al. 2006; Perez et al. 2008a, b; Seidman et al. 2002; Tan-Chiu et al. 2005). Hence, increased cardiac stress seems to increase the risk of trastuzumab associated cardiac side effects, which may explain why it appears to be more frequent in clinical practice than in controlled trials (McArthur and Chia 2007). Trastuzumab cardiotoxicity is not dose related, and it is frequently reversible. However, if combined with anthracyclines, the frequency and risk of anthracycline induced myocyte death might increase (Zuppinger et al. 2007).
4.1.2.6 Small Molecule Tyrosine Kinase Inhibitors
Lapatinib is used in the treatment of disseminated breast cancer. It has been reported to be associated with symptomatic (grade 3 or 4 heart failure) cardiotoxicity in 0.2 % of patients and with asymptomatic events (>20 % decrease in left ventricular ejection fraction without symptoms) in 1.4 % of patients (Perez et al. 2008b). The risk was increased in patients with prior anthracycline treatment (2.2 %) or prior trastuzumab treatment (1.7 %). The mean time to onset of symptoms was 13 weeks. Lapatinib has also been reported to cause QT prolongation in 16 % of patients treated with varying doses (Yeh and Bickford 2009). Lapatinib thus seems to be less cardiotoxic than trastuzumab. This may partly be due to selection bias in the trials of patients receiving lapatinib, but recent data indicate an underlying molecular mechanism for the apparent difference in cardiotoxicity, as lapatinib, but not trastuzumab protects against TNF-alpha-induced cardiomyocyte death (Spector et al. 2007).
Imatinib, used in the treatment of chronic myeloid leukemia and in gastro-intestinal stromal tumors, has been associated with rare heart failure (Kerkela et al. 2006). The incidence of clinical heart failure in patients treated with imatinib has been reported as 0.5–1.7 % (Atallah et al. 2007; Hatfield et al. 2007). Whether the imatinib-associated cardiotoxicity is reversible or not is still unknown.
Dasatinib, also used in the treatment of chronic myeloid leukemia, has been reported to be associated with heart failure in 2–4 % of patients, and QT prolongation has been reported to occur in 2–3 % of patients treated with dasatinib (Yeh and Bickford 2009).
Sunitinib, simultaneously approved for the treatment of renal cell carcinoma, and gastrointestinal stroma tumor, has been associated with left ventricular dysfunction in 4–11 % of patients, with symptomatic heart failure in 2.7–8 % (Chu et al. 2007; Khakoo et al. 2008). The mean time to the development of heart failure varied between 22 days and 27 weeks, and the only risk factor was prior coronary artery disease. Although heart failure responded to medical therapy, it did not seem to be fully reversible (Khakoo et al. 2008). Moreover, sunitinib has been associated with hypertension in 5 to 47 % (Chu et al. 2007), in different clinical trials, with grade 3 occurring in 2–8 % (Chu et al. 2007; Burstein et al. 2008; Demetri et al. 2006; Motzer et al. 2006a, b, 2007; Azizi et al. 2008).
Sorafenib, approved for the treatment of advanced renal cell carcinoma and hepatocellular carcinoma, causes hypertension in a significant number of patients. The overall incidence in a recent meta analysis was 23.4 %, with grade 3 and 4 reported in 2.1–30.7 % in different studies (Wu et al. 2008). Sorafenib has also been associated with myocardial ischemia in 3 % of patients in clinical trials, which is significantly higher than in patients treated with placebo (Yeh and Bickford 2009; Escudier et al. 2007).
Kinase Inhibition Determines TKI-induced Toxicity to Normal Tissue:
Cardiotoxicity is NOT a class effect for TKIs.
Cardiotoxicity determined by the specific kinases that a TKI inhibits (whether on-target or off-target).
Small molecule TKIs inherently inhibit many different kinases than monoclonal antibodies.
Multi-targeted TKIs inhibit more kinases (i.e., sunitinib > imatinib).
Certain inhibited kinases are essential to tumor cell killing and also turn out to be necessary for cardiomyocyte survival.
Constant balance between enhanced tumor cell killing versus potential increased risk of cardiotoxicity.
4.1.2.7 Vorinostat
Vorinostat is approved for the treatment of cutaneous T cell lymphomas. Vorinostat had been reported to be associated with QT prolongation in 3.5–6 % of patients (Yeh and Bickford 2009; Olsen et al. 2007). Risk factors are female gender, older age, previous myocardial ischemia, heart failure, electrolyte imbalances, bradycardia, and medication with QT prolonging drugs (Yeh and Bickford 2009; Vorchheimer 2005).
4.1.2.8 Thalidomide
Thalidomide is used in the treatment of multiple myeloma. Bradycardia has been associated with thalidomide treatment in 2–55 % of patients. Most patients are asymptomatic, but some patients may experience fatigue, syncope, or dizziness. Patients developing third-degree atrioventricular block need a permanent pacemaker.
4.1.2.9 Arsenic Trioxide
Arsenic trioxide is used in the treatment of certain leukemias. QT prolongation has been reported in patients treated with arsenic trioxide in 26–93 % of patients (Barbey et al. 2003; Huang et al. 1998; Ohnishi et al. 2002; Shigeno et al. 2005; Singer 2001; Unnikrishnan et al. 2004; Westervelt et al. 2001). The QT interval has been reported to be prolonged from 1 to 5 weeks after treatment and then returned to baseline (Soignet et al. 2001).
4.1.2.10 Toxic Effect of Systemic Therapy on Coronary Arteries
Direct effects of chemotherapy on coronary arteries are rare.
Fluoropyrimidines (i.v. 5 fluoro-uracil (5FU) or oral capecitabine), may cause myocardial ischemia and decreased contractility of the heart (Kosmas et al. 2008; Tsibiribi et al. 2006a, b; Manojlovic et al. 2008). A spasm of the coronary arteries is often considered to be the most important cause. The underlying mechanism is not fully elucidated yet. Experimental studies in rabbits indicate that a spasm of the coronary arteries is not the only mechanism of 5FU cardiotoxicity, and that apoptosis of myocardial and endothelial cells can result in inflammatory lesions mimicking toxic myocarditis (Tsibiribi et al. 2006a, b).
4.1.3 Effects of Combined Modality Treatment on the Heart
Whether toxicity following chemotherapy and radiotherapy are additive or synergistic is still unclear. Several clinical studies in lymphoma patients showed that anthracycline-containing therapy may further increase the radiation-related risk of congestive heart failure and valvular disorders by 2- to 3-fold compared to radiotherapy alone (Aleman et al. 2007; Moser et al. 2006). A Dutch study including 5-year survivors of Hodgkin lymphoma treated before age 41 showed that the 25-year actuarial risks of heart failure after mediastinal radiotherapy alone versus anthracycline-based chemotherapy in combination with mediastinal radiotherapy were 7.5 versus 10.7 % respectively (Aleman et al. 2007).
Myrehaug et al. 2008 evaluated the risk of hospital admission for cardiac disease in Hodgkin lymphoma patients, adjusting for age, sex, treatment, cardiac risk factors, and competing causes of death. They showed that for females and males treated with doxorubicin plus mediastinal radiotherapy at age 40, the estimated 15-year incidence rate of cardiac hospitalization was 7.3 and 16.5 %, respectively, rates 5–15 % higher than expected. They also showed that the cardiotoxic effects of radiotherapy and chemotherapy may be more than additive.
A prospective study in breast cancer patients compared 10 versus 5 cycles of doxorubicin (A) (45 mg/m2) and cyclophosphamide (C) (500 mg/m2) chemotherapy. In retrospective subgroup analysis of patients treated with radiotherapy, an increased rate of cardiac events was found among patients receiving 10 cycles of chemotherapy and radiotherapy (Shapiro et al. 1998), with a 6-year median follow-up.
Trastuzumab and other systemic agents (e.g., taxanes and biologicals) will be used more commonly along with thoracic radiotherapy in patients. There is only limited data on possible interactions between these agents and radiation with respect to cardiotoxicity. In a recent multicenter, prospective trial assessing the utility of trastuzumab in breast cancer, the concurrent use of trastuzumab along with left-sided RT appeared to be safe, albeit with a follow-up of only 3.7 years (Halyard et al. 2009) Dose-volume data regarding the degree of heart irradiation were not reported; though purposeful irradiation of the internal mammary nodes was not permitted.
4.2 Toxic Effects on Other Blood Vessels
Arteries may be damaged by radiation. Veins seem to be less vulnerable to radiation effects. The clinically most important arterial radiation-related problems are mentioned below.
4.2.1 Carotid Arteries
Damage to the carotid arteries is of special importance. Significantly, increased risks of stroke have also been described in patients treated with radiotherapy for head- and-neck cancer (60–70 Gy; RR 2.1–9.0, depending on follow-up) (Dorresteijn et al. 2002; Haynes et al. 2002; Scott et al. 2009), Hodgkin lymphoma (median dose approximately 40 Gy; RR 2-to 4-fold increased) (Bowers et al. 2005; De Bruin and Dorresteijn 2009) and in long-term survivors of childhood leukemia and brain tumors treated with >30 Gy cranial radiotherapy (RR 5.9 and 38, respectively) (Bowers et al. 2006). The latter study also demonstrated a relationship between radiation dose and RR stroke, with significantly higher risks for cranial doses of >50 Gy compared with 30–50 Gy.
In a Dutch retrospective cohort study of 2,201 5-year survivors of Hodgkin lymphoma treated before age 51 between 1965 and 1995, there was a substantially increased risk of stroke and TIA, associated with radiation to the neck and mediastinum (De Bruin and Dorresteijn 2009). Most ischemic events were from large artery atherosclerosis (36 %) or cardioembolisms (24 %). The standardized incidence ratio for stroke was 2.2 (95 % confidence interval [CI] = 1.7–2.8), and for TIA, it was 3.1 (95 % CI = 2.2–4.2). The risks remained elevated, compared with those in the general population, after prolonged follow-up. Treatment with chemotherapy was not associated with an increased risk. Hypertension, diabetes mellitus, and hypercholesterolemia were associated with the occurrence of ischemic cerebrovascular disease, whereas smoking and overweight were not.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree