Chapter 8 Coronary Computed Tomography Angiography
INTRODUCTION
Noninvasive cardiac imaging is of vital importance for the assessment of coronary artery disease.
TECHNICAL DEVELOPMENT OF SCANNERS
If the development of cardiac CT over the past decade is reviewed, it is noted that cardiac imaging was possible with 4-slice systems, but most research studies that were conducted removed a substantial number of coronary artery segments (up to 30%) from their analysis because of nonevaluability. This was in part as a result of presence of motion artifact (image blurring). The reason for the large amount of motion artifact with these systems is the slower gantry rotation speed of approximately 500 ms (resulting in a temporal resolution of 250 ms). The major improvement with 64-slice CT systems was in the reduction of the number of nonevaluable segments, which is in keeping with the improved temporal resolution. Typical gantry rotation speeds of 16-slice scanners are 420 ms to 370 ms (even though some remained at 500 ms at first), resulting in temporal resolutions typically ranging from 210 ms to 185 ms. This has caused the number of nonevaluable coronary artery segments to go down to on average approximately 6%. Keep in mind that temporal resolution and the resulting motion artifact is only one of the two major reasons for unevaluable segments, the other one being presence of dense coronary calcium. The next major step in the development of cardiac CT was the 64-slice MDCT generation. All major vendors have offered a 64-slice MDCT system, even though the number of slices is calculated in different ways. Some vendors have actually 64 equally sized detector rows within the gantry and have x-rays emitted from one focal spot in the x-ray tube. One vendor, however, uses 32 equally spaced detectors and two focal spots on the x-ray tube that alternate in emitting x-rays. Thus they acquire two different projections for each of the 32 detector rows, resulting in 64 individual projections.
TECHNICAL PRINCIPLES
Retrospective Gating versus Prospective Triggering
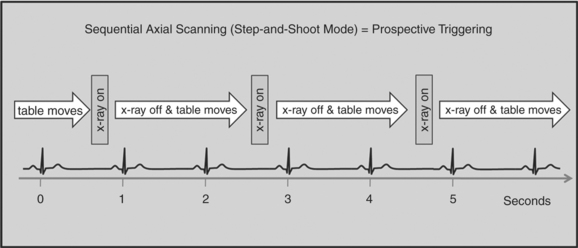
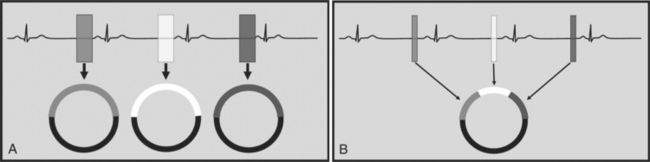
There are two general approaches to cardiac synchronization of the CT acquisition. One is prospective and “observes” the electrocardiogram (ECG) for a small number of heart beats (or more accurately the peak of the R-wave or R-peak) and then anticipates when the next R peak is to occur. Given the anticipated time point of the future R peak, the scanner will then only acquire x-ray projections in a prespecified phase of the cardiac cycle (usually late in diastole where the heart is most quiescent). This approach is called prospective triggering because the x-ray tube is triggered to shoot in a predefined cardiac phase. Data acquisition is in an axial fashion, and the table only moves in between heartbeats and is stationary during x-ray transmission. This approach has the advantage of having a low radiation dose to the patient, but it has a number of disadvantages. One of the major disadvantages is that typically only one dataset (or few similar ones) can be acquired in the anticipated cardiac cycle, which may not turn out to be of optimal image quality (Fig. 8-1).
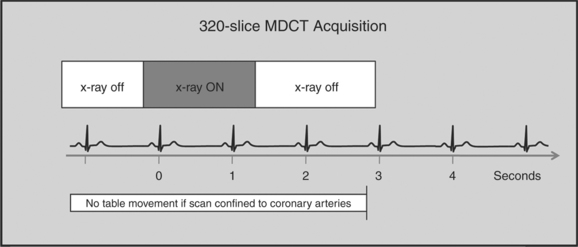
The newer 256- and 320-slice scanners use a modified step-and-shoot mode. Because their detectors cover a large volume, in many cases the entire heart, no table motion is necessary to acquire a coronary CTA dataset. Having the tube current turned on for approximately one entire heartbeat allows acquiring a dataset for analysis of cardiac anatomy and function. Theoretically, x-ray exposure can be limited to a short segment in diastole if only coronary artery visualization, and if no information on function is desired. Multi-phase reconstruction (to improve temporal resolution) would, however, require data acquisition during several consecutive cardiac phases (Fig. 8-2).
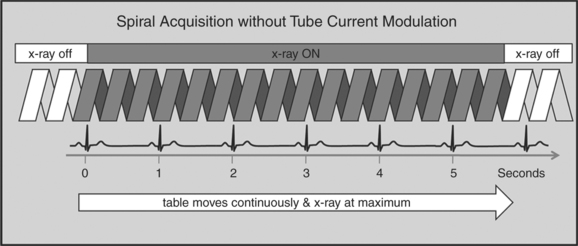
A radically different approach is retrospective gating (Fig. 8-3). Retrospective gating allows acquiring unlimited complete datasets in any phase of the cardiac cycle. This approach uses a spiral CT acquisition, in which the x-ray current remains turned on during the entire scan. The user may then in retrospect define what phase of the cardiac cycle to reconstruct. The major advantage of this approach is that the interpreter may decide to try a different phase of the cardiac cycle if the initial reconstruction demonstrates motion artifact. Another advantage is the ability to “edit” the ECG. ECG editing allows the user to select heartbeats that should not be used for reconstructions (e.g., premature ventricular contractions [PVCs]), or to correct trigger points that were not placed on an R peak by the computer algorithm. The major disadvantage of retrospective gating is the high radiation dose to the patient. For this reason a number of dose reduction strategies were developed.
Tube Modulation
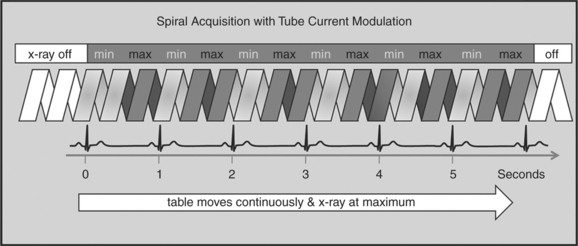
One of the most important dose reduction strategies in cardiac CT is ECG-correlated x-ray tube current modulation or short tube modulation. In this algorithm the scanner does perform a spiral acquisition using the retrospective gating method; however, it prospectively down regulates the x-ray tube current (typically in systole and usually down to approximately 20% of the maximum). This reduces the radiation dose to the patient during systole, but nevertheless allows for reconstructions of images in systole, if necessary. The penalty for reducing the tube current is a higher noise level. This approach is clearly a compromise trying to capture the advantages of both the prospective triggering and the retrospective gating acquisitions (Figure 8-4).
HALF-SCAN VERSUS MULTI-SEGMENT RECONSTRUCTION
To capture images without blurring from rapid cardiac motion, it is important to achieve a high temporal resolution. Temporal resolution refers to the time it takes to collect all the data (projections) to generate an axial source image. The time it takes to collect these data (the temporal resolution) is dependent on how fast the CT gantry spins around the patient. In conventional CT, the temporal resolution is equal to the gantry rotation speed. There are, however, ways to improve the temporal resolution for cardiac CT imaging. Two algorithms can be applied, the “half-scan” reconstruction algorithm, or the “multi-segment reconstruction” algorithm (Fig. 8-5A, B).
SCAN ACQUISITION
Contrast Injection
There are a number of injection protocols that can be applied to coronary CTA. It is beyond the scope of this chapter to review all these. However, there are a number of common principles that are important to review. The intravenous catheter is ideally of large bore and placed in the right antecubital fossa. The right-sided injection is somewhat preferred because of a shorter route to the heart and because it avoids the crossing of dense contrast past the left internal mammary artery (LIMA) and the great vessels via the left innominate vein. This has the potential to cause streak artifact, which, for example, can hinder the assessment of a portion of a LIMA graft.
CORONARY ANATOMY
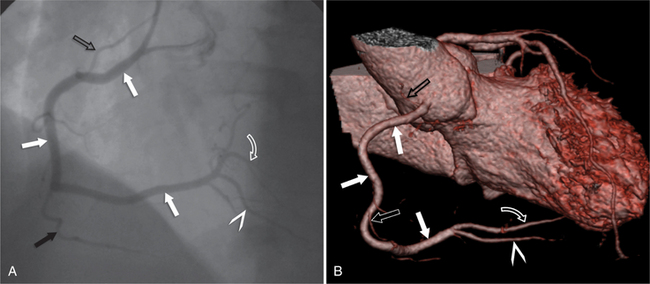
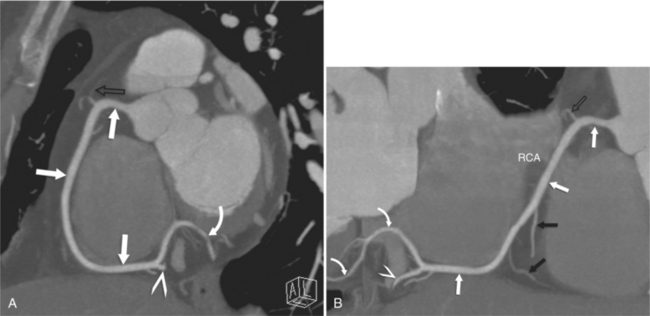
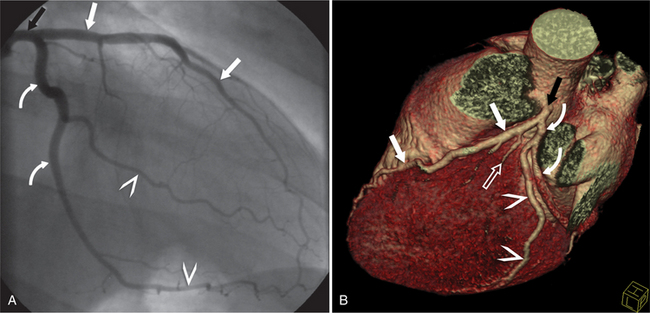
The RCA originates from the right sinus of Valsalva. The first RCA branch is the conus branch, which supplies the myocardium of the right ventricular outflow tract (RVOT). Occasionally the conus branch may have a separate ostium from the right sinus. The RCA gives rise to anterior right ventricular (RV) free wall branches and acute marginal branches that run along the angle that the anterior and inferior RV free walls form. The RCA is dominant in ≈80% of cases and runs in the right atrioventricular groove up to the crux of the heart (the point of the inferior cardiac surface where the atria and ventricles meet), where it bifurcates into a posterior descending artery (PDA) that runs within the inferior interventricular groove, and a posterior left ventricular branch (PLV) that supplies the inferior left ventricular (LV) wall (Figures 8-6, 8-7, 8-8). The PLV often gives rise to a small atrioventricular nodal branch at the crux of the heart. If the RCA is nondominant, it usually does not reach the crux of the heart, and the PDA and PLV are supplied by the left circumflex coronary artery (LCX).
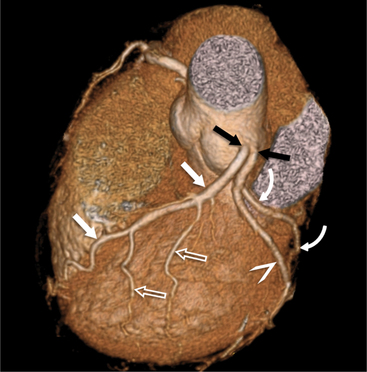
The left main coronary artery (LM) origin is usually more cephalad compared to the RCA ostium. The LM originates from the left sinus of Valsalva and bifurcates within 2 cm of its origin into the left anterior descending artery (LAD) and LCX (Figures 8-9, 8-10). Occasionally there is no LM, and the LAD and LCX both originate directly from the left sinus of Valsalva (Fig. 8-11). An LM trifurcation is a situation in which there is a third branch arising from the LM between the LAD and LCX (Fig. 8-12). This branch is called ramus intermedius.
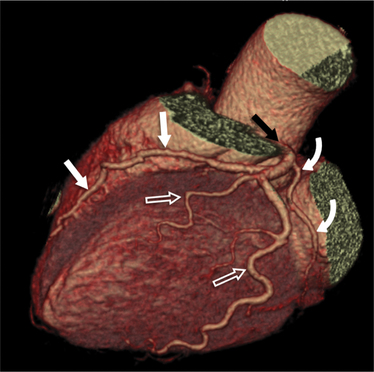
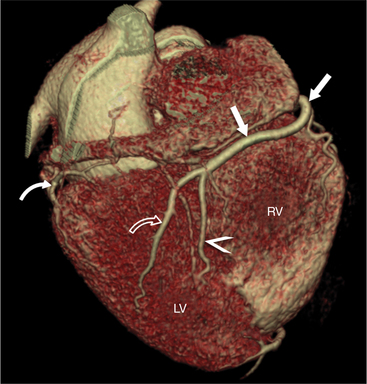
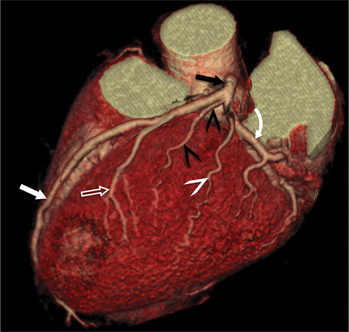
FIGURE 8-10 Normal coronary anatomy. Volume-rendered reconstruction of coronary computed tomography angiography shows the opposite end of the spectrum of normal (compared to Figure 8-9) with a large branching first diagonal branch (open white arrows) and a small left circumflex artery with no obtuse marginal branches. Note the left anterior descending artery (white arrows).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree