- •
Position of the morphological right ventricle on the left, giving rise to the aorta, and the morphological left ventricle on the right, giving rise to the pulmonary artery.
- •
Left-sided tricuspid valve abnormalities such as “Ebstein-like” appearance, valve thickening, dysplasia, and/or valvar insufficiency.
- •
Presence or absence of ventricular septal defects.
- •
Right-sided left ventricular outflow tract obstruction as either subpulmonic or pulmonary valve stenosis.
- •
Left-sided right ventricular hypoplasia and possible hypoplasia of the aorta or coarctation of the aorta.
- •
In the presence of a large ventricular septal defect, straddling or overriding of the atrioventricular valves.
- •
Conduction disorders, heart block (look for bradycardia or asynchrony between contraction of the atria and ventricles).
Anatomy and Anatomical Associations
Corrected transposition of the great arteries (cTGA) is a cardiac defect in which there is atrioventricular (AV) discordance and ventriculoarterial concordance. What this means is as follows.
In the setting of atrial situs solitus, the right atrium connects via the mitral valve to a right-sided morphological left ventricle, which then ejects into the pulmonary artery. In turn, the left atrium connects via the tricuspid valve to the left-sided morphological right ventricle, which ejects into the aorta. If one defines “transposition of the great arteries” as the aorta arising from the right ventricle and the pulmonary artery from the left ventricle, this condition is achieved in cTGA. However, because the ventricles, not the great vessels, are switched, in effect, the systemic venous return (deoxygenated blood) makes its way to the lungs via the right atrium–to–left ventricle–to–pulmonary artery connection and the pulmonary venous return (oxygenated blood) makes its way to the body via the left atrium–to–right ventricle–to–aorta connection ( Figure 15-1 ). There is “physiological correction” because the normal pathways of deoxygenated blood to the lungs and oxygenated blood to the body persist in the correct manner.
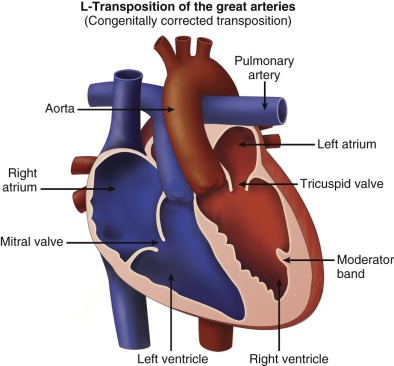
In cTGA, the aorta is anterior and leftward of the pulmonary artery. The cardiac segments are as follows: atrial situs solitus (S), l -ventricular loop (L), l -transposition of the great arteries (L) or {S,L,L} in the Van Praagh nomenclature. cTGA can also occur in the setting of atrial situs inversus with levocardia or with mirror-image dextrocardia. The segments in these arrangements are situs inversus, d -loop ventricles, and d -transposition of the great arteries {I,D,D}.
Only approximately 10% of cTGA occurs in isolation; this defect is most often associated with other intracardiac abnormalities. Dextrocardia or mesocardia is present in 25% of patients. Atrial situs inversus is found in 3% to 5%. Ventricular septal defect (VSD) is the most common association, present in 70% to 80% of cases of cTGA. The majority of these defects involve the membranous septum owing to the misalignment of the atrial and ventricular septa usually seen in cTGA. They are often large and extend anteriorly.
Autopsy studies have shown over 90% of cases of cTGA are associated with anomalies of the tricuspid valve. Approximately 25% patients with cTGA have an Ebstein-like malformation of the left-sided tricuspid valve where the septal leaflet is significantly displaced below the level of the annulus. Other tricuspid valve anomalies include dysplastic leaflets and straddling across the VSD. These anomalies can result in significant tricuspid regurgitation.
Right-sided left ventricular or pulmonary outflow tract obstruction occurs in 40% to 50% of patients with cTGA and is usually found at the subvalvar level as a result of fibrous tissue protruding into the outflow tract. Pulmonary atresia is found in 15% of patients. Less commonly, mitral valve abnormalities occur in 10% and right ventricular outflow tract obstruction and aortic arch obstruction are present in 13%.
Patients with cTGA also have abnormalities in their conduction system. The sinoatrial node is in the normal position. However, the AV node with its conducting bundle is situated anteriorly in the interatrial septum, on the right side. In some patients, there is an additional, hypoplastic AV node found in the normal posterior position. It has been shown that dual AV nodes are most often found in cTGA patients with significant AV septal misalignment. The abnormal positioning of the AV node and the fibrosis noted in this elongated node explain the relatively high percentage of AV block and other arrhythmias found in patients with cTGA.
In cTGA with l -looped ventricles, the left main coronary artery arises from the anterior-rightward facing sinus and the right coronary artery arises from the posterior-facing sinus. Coronary anomalies are rare in cTGA when compared with d -transposition of the great arteries.
Frequency, Genetics, and Development
cTGA occurs in approximately 0.03 per 1000 live births and accounts for less than 1% of all congenital heart disease. Clearly, a multifactorial etiology must be considered. Environmental factors have been documented in some cases. Epidemiology studies have demonstrated the recurrence risk in siblings of patients with congenital heart disease to be approximately 1% to 3%. One study involving 102 patients specifically with cTGA demonstrated a recurrence risk of congenital heart defects in 5% of their siblings. More recent molecular studies have given important consideration to gene abnormalities involved in congenital heart defects, but specific gene abnormalities in cTGA have yet to be reported.
As early as day 15 of human embryological development, the heart field begins as a crescent-shaped group of cells. By day 21, the heart is a long tube with the sinus venosus on one pole and the conotruncus on the other. Normally, the tube loops to the right ( d -looping) to ensure the proper positioning of the right and left ventricles. In cTGA, the tube loops to the left ( l -looping). This abnormal looping, in turn, disturbs the normal process by which the conotruncus septates, twists, and rotates to ensure normal ventricular-arterial connections.
Fetal Physiology
In fetuses with cTGA and no other significant associated defects, the physiology is equivalent to a normal fetal circulation. cTGA with a VSD results in fetal physiology similar to that of an isolated VSD. Bidirectional shunting is usually seen in utero. The presence of unidirectional shunt indicates outflow tract obstruction contralateral to the direction of shunting. Significant pulmonary or aortic outflow tract obstruction in the setting of cTGA may result in a ductal-dependent circulation. Fetuses generally tolerate obstruction well in utero as long as the ductus arteriosus is widely patent.
In cTGA, the left ventricle situated on the right side takes on the task of dominant perfusion through ejection into the ductus arteriosus to the descending aorta, and the right ventricle situated on the left performs the task of perfusion into the aorta and head and neck vessels. This shifting of duties between the ventricles is well tolerated in the fetus, without consequence, as long as the AV valves function well.
Fetuses with cTGA and an Ebstein-like malformation of the tricuspid valve may develop significant tricuspid valve regurgitation. The resulting increase in atrial pressure can lead to fetal hydrops with cardiomegaly, decreased ventricular shortening, pericardial and pleural effusions, and arrhythmias. Severe hydrops fetalis in this scenario commonly leads to fetal demise. If these fetuses survive, they are at high risk of significant complications after birth. In addition, there are reports of severe tricuspid valve regurgitation leading to decreased forward flow through the aorta in fetal life and the development of aortic arch obstruction.
As high as 20% of fetuses with cTGA have varying degrees of AV block and associated bradycardia. Heart block may be present at the first prenatal evaluation or develop over gestation. Rarely, supraventricular tachycardia has been noted.
Prenatal Management
The fetal echocardiogram demonstrates the cardiac segmental anatomy, AV discordance, transposition of the great arteries, and the presence of the associated anomalies outlined previously.
After the right and left side of the fetus is established, a cross-sectional view of the abdomen will aid in the determination of situs by noting on which side of the spine the aorta and the inferior vena cava lie. If the aorta is to the right and the inferior vena cava is to the left of the spine, situs inversus is present.
A cross-sectional view of the chest with the four chambers of the heart in view will demonstrate the position of the heart within the chest (levocardia, mesocardia, or dextrocardia). Usually, in this view of the heart when imaging posteriorly at the level of the inflows, atrial situs can be confirmed by visualization of pulmonary vein connections to the left atrium. In turn, the connection of the left atrium to the morphological right ventricle can be seen in this view (features of the right ventricle include the presence of a moderator band, coarse trabeculations, triangular-shaped cavity, and a more apically displaced tricuspid valve with direct attachments to the ventricular septum), confirming the ventricular looping abnormality in cTGA. A sweep of the ventricular septum from posterior to anterior may also reveal significant VSDs.
Morphologic assessment of the tricuspid valve by two-dimensional imaging and color Doppler interrogation for regurgitation can be performed in this view. Details of mitral valve morphology can also be obtained. An anterior sweep from this four-chamber view to the level of the great vessels confirms the ventricular-arterial discordance and will demonstrate parallel exit of the great vessels with the aorta anterior and to the left of the pulmonary artery in the setting of normal atrial situs. Assessment of outflow tract obstruction and the mechanism of obstruction (valvar, subvalvar muscle or membrane, accessory AV valve tissue) may be determined in this view. In the setting of severe outflow tract obstruction, an element of ventricular hypoplasia may be present. Ventricular size discrepancy can be appreciated in this four-chamber view.
The abnormal great vessel relationship and the size of the great vessels relative to each other can also be confirmed using the three-vessel view. From the four-chamber view, a sweep superiorly moving leftward normally demonstrates cross-sectional views of the right superior vena cava, followed by the aorta, followed by the pulmonary artery in order from posterior and rightward to anterior and leftward ( Figure 15-2A ). In cTGA in such a sweep, the right superior vena cava is followed by the pulmonary artery, which is situated posterior, and then the aorta, which is situated anterior and leftward to the pulmonary artery (see Figure 15-2B ). Usually, the aorta is slightly smaller than the pulmonary artery. If the aorta is the same size or larger, this discrepancy may indicate pulmonary outflow obstruction. Often, the direction of flow in the ductus arteriosus can be assessed in this view along with the branch pulmonary artery size.