Robert L. Bard, Jurgen J. Fütterer and Dan Sperling (eds.)Image Guided Prostate Cancer Treatments201410.1007/978-3-642-40429-0_1
© Springer-Verlag Berlin Heidelberg 2014
1. Current Scope of Prostate Cancer
(1)
Bard Cancer Center, Biofoundation for Angiogenesis Research and Development, 121 E. 60th St, New York, NY 10022, USA
Abstract
Prostate cancer statistics and ambivalence about screening challenge our profession to enlarge our array of clinical practices in order to better serve patients’ needs. The diagnostic and treatment conventions that dominated urologic practice at the turn of the millennium are rapidly evolving toward an image-guided target-and-manage approach to the disease. Advances in imaging coupled with biomarkers enable more informed decisions about the timing and utilization of diagnostic and treatment interventions. In addition to advanced imaging technologies, complementary and alternative medicine also offer hope for preventing, managing, and overcoming prostate cancer. The overall statistics for prostate cancer challenge our profession to serve patients’ needs by enlarging our array of clinical choices.
1.1 Introduction
Prostate cancer statistics and ambivalence about screening challenge our profession to enlarge our array of clinical practices in order to better serve patients’ needs. The diagnostic and treatment conventions that dominated urologic practice at the turn of the millennium are rapidly evolving toward an image-guided target-and-manage approach to the disease. Advances in imaging coupled with biomarkers enable more informed decisions about the timing and utilization of diagnostic and treatment interventions. In addition to advanced imaging technologies, complementary and alternative medicine also offer hope for preventing, managing, and overcoming prostate cancer.
1.2 The Changing Scope of Prostate Cancer
The revered Ewing’s textbook of pathology Neoplastic Diseases (Ewing 1940) mentioned that prostate cancer was a rare disease comprising about 0.27 % of overall male cancers. We now know this is not the case. In fact, prostate cancer (PCa) is the most common cancer in North America. It is an expensive disease, representing 11 % of the costs for the treatment of all cancers in the USA with a per-patient cost from diagnosis to death of $81,658 for a total of $8 billion annually (Racioppi et al. 2012). While the price tag of a single preferred treatment may limit a patient’s access to it, the costs strain the overall economics of private and public healthcare administration.
A less public but perhaps more poignant burden is borne by the patient and his family. The burden contains varying contents depending on the individual patient: whether or not to screen, TRUS biopsy with its attendant flaws and risks, treatment cost/benefits analysis, seeking second opinions and conducting extensive internet research, decision-making, undergoing treatment, recovery issues, and impact on quality of life. As recently as three decades ago, many patients were paying an even higher price in terms of late-stage diagnosis, radical localized treatments with high comorbidity rates despite the increased odds of undetected metastatic disease, suffering from late-stage systemic disease, and death. Thankfully, a simple blood test for prostate-specific antigen (PSA) reversed the damages of late-stage diagnosis, but it also sparked a downward trend in age at the time of diagnosis and created new complications from overtreatment of low-grade indolent cancers.
The identification of prostate-specific antigen (PSA) as a possible prostate cancer (PCa) marker resulted from the work of various researchers throughout the 1960s and 1970s. In 1986, the FDA approved the PSA blood test to monitor the progression of prostate cancer (PCa) in men already diagnosed with the disease. Though the FDA did not approve the use of this test, in conjunction with the digital rectal exam, to screen asymptomatic men until 1994, the 6 years from 1986 to 1992 saw a doubling of incidence of PCa in the male population and a sharp shift of the diagnosis to a younger age group.
1.2.1 The Prostate Cancer Screening Dilemma
With widespread screening and increased grassroots awareness, there occurred a significant trend toward detection of clinical stage T1c prostate cancer (from 2.1 to 36.4 %) while those with palpable clinical stage T3 decreased from 23.5 to 6.5 % (Amling et al. 1998). Thus, there were far fewer cases of metastases at diagnosis. In the USA over the past 20 years, prostate cancer death rates have been reduced by close to 40 % without substantial changes in surgical or radiation treatment strategies. Some models suggest that more than 50 % of this reduction is due to early detection (Scosyrev and Messing 2013).
In addition to earlier detection, a demographic shift occurred. While the overall prostate cancer incidence rate was stable from 2001 to 2007, rates significantly increased among men aged 40–49 years and decreased among men aged 70–79+ years. In addition, about 42 % of localized prostate cancers diagnosed from 2004 to 2007 were poorly differentiated (Li et al. 2012).
Therefore, the apparent increase in survival advantage due to screening and the raised stakes for younger patients who may have aggressive disease would seem to warrant continued screening despite its imperfections.
On the other hand, in 2012, the U.S. Preventive Services Task Force (USPSTF) recommended against routine PSA screening for prostate cancer due to the risk of overdiagnosis and overtreatment with most prostate cancer remaining asymptomatic. The panel concluded that the potential benefit of testing did not outweigh the risk of harm, arguing against continued screening except for those with known risk factors and a life expectancy greater than 10 years.
Mixed reviews came in from the European Randomized Study of Screening for Prostate Cancer (ERSSPC). The study concluded that while PSA-based screening indeed reduced the rate of death from prostate cancer by an estimated 20 %, it was associated with a high risk of overdiagnosis (diagnosis in men who would not have clinical symptoms in their lifetime) (Schröder et al. 2009). In fact, overdiagnosis, overtreatment, and its concomitant comorbidities are likely the most important adverse effects of screening and are significantly more common than in screening for breast, colorectal, or cervical cancer (Hakama and Auvinen 2008). Knowing this, a younger PCa patient might be deterred from screening by the risk of posttreatment erectile dysfunction.
To screen or not to screen? The approach-avoidance dilemma over this issue is understandable if PSA, DRE, and TRUS biopsy are the only tools available to physicians and patients. However, advances in imaging and precision tumor ablation are spurring clinicians to adopt new practice models that overlap and integrate urology and radiology.
1.3 Limitations of PSA Testing and an Alternate Paradigm
Modern thinking regarding prostate cancer screening is shaped by major US and British studies pointing to a need for a better diagnostic test than random biopsies for men with elevated PSA. Prostate cancer is currently the only malignancy that is diagnosed using a nonspecific blood test leading to a random set of biopsies of the entire organ. Since only 3 % of prostate cancer is lethal, a new paradigm for diagnosing and treating prostate cancer has evolved based on the following assumptions:
1.
Gleason 3 + 3 tumors do not metastasize (Lepor 2013) and have a 1 % overall mortality at 15 years, meaning curative radical treatment may be replaced by more conservative approaches.
2.
Metastatic lesions appear to be attributable to a single clone of cells that form a significant or dominant lesion.
3.
Imaging modalities such as high-resolution sonography and advanced MR imaging protocols usually find the dominant tumor.
4.
Focal dominant cancers may be ablated under image guidance of ultrasound (US) and/or MRI.
5.
Untreated patients will live as long as treated patients.
6.
Focal treatment is a targeted therapy with a reduced economic burden and less risk of injury to the periprostatic structures of the neurovascular bundles, rectum, bladder, and urinary sphincter. Focal ablation is an alternative to active surveillance/watchful waiting and radical prostatectomy/radiation therapies.
1.3.1 Prostate Imaging: Resolving the Screening Dilemma?
Advanced imaging may provide a fundamentally organic solution to the screening dilemma by both avoiding unnecessary biopsies and also minimizing the burdens associated with overtreatment.
1.
Is a biopsy warranted? When biomarkers raise a suspicion of PCa, imaging can identify a region of interest (ROI) to help determine if a biopsy is warranted. Thus, imaging abrogates the rush to a randomized, systematic biopsy, which entails risks of minor complications (60–79 %) and sampling challenges in all zones (Carroll and Shinohara 2008).
2.
Is a focal treatment reasonable for clinically qualified patients? Precise image-guided ablation of all known lesions can “favorably alter the natural history of the cancer without impacting health-related quality of life and allows for safe retreatment with repeated focal therapy or whole-gland approaches if necessary” (Mearini and Porena 2011).
3.
Is active surveillance a viable option for men with early-stage, low-risk disease? Imaging provides a way to monitor for disease progression, allowing a patient to defer the treatment decision and maintain, or even improve, quality of life and health through lifestyle changes that may also help manage his cancer.
1.3.2 Active Surveillance
Ninety-seven percent of men with tumors will die from other causes, calling into question the rush to treat biopsy-demonstrated PCa. Patients with early-stage, low-risk disease are often considered candidates for what was historically termed watchful waiting (WW). Today’s preferred nomenclature is active surveillance (AS), since lifestyle changes and antioxidant treatments bring dynamism to this option that addresses both the overdiagnosis caused by PSA screening and the potential side effects of the standard treatments of radical prostatectomy (impotence and incontinence) and radiation therapy (bladder and gastrointestinal toxicity) (Wei et al. 2002).
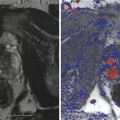
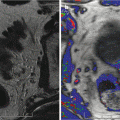
Men with organ-confined disease that is low risk and who can tolerate the anxiety of deferring standard treatment are candidates. The initial definition of insignificant prostate cancer 4 (Stamey et al. 1993) was Gleason grade <7, PSA < 10 ng/ml, and physical disease volume of 0.5 cc. This has been generally replaced by the definition of significant cancer as volume >1.3 cc, Gleason score >6, and a dominant lesion imaged by high-resolution Doppler ultrasound and/or multiparametric MRI (Sauvain et al. 2013).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree