Advanced pulmonary disease continues to remain the leading cause of morbidity and mortality in patients with cystic fibrosis (CF), with pulmonary imaging playing a crucial role in early detection, longitudinal monitoring, as well as prelung and postlung transplant evaluation. This article reviews the specific imaging features of CF using conventional imaging modalities (chest radiographs and high-resolution computed tomography [HRCT]) as well as emerging imaging technologies (digital chest tomosynthesis and MR imaging). In addition, the authors review the CF-specific HRCT imaging findings that are essential in the evaluation of these patients in the pre–lung transplant and post–lung transplant settings.
Key points
- •
Radiographs and high-resolution computed tomography (HRCT) continue to be the primary imaging modalities in the initial assessment and longitudinal monitoring of cystic fibrosis (CF).
- •
Recently, emerging imaging modalities, including digital chest tomosynthesis and magnetic resonance imaging, have shown promise in the evaluation of CF with their exact roles yet to be defined.
- •
With an increasing number of patients with CF receiving lung transplantations, it is crucial for the radiologist to be aware of the CF-specific pre-transplant and post-transplant HRCT findings when assessing these patients.
Introduction
Cystic fibrosis (CF) is the most common lethally inherited disease in Caucasians with approximately 30,000 affected individuals in the United States alone, as of 2017. , Characterized by mutations resulting in dysfunctional chloride channel ion transporters, CF is a multisystem disorder with pulmonary, pancreatic, hepatobiliary, gastrointestinal, reproductive, osseous, and infectious manifestations. Of these complications, advanced pulmonary disease dominates the clinical landscape and results from abnormal mucociliary clearance with subsequent episodes of chronic bronchopulmonary infection, mucus obstruction, and eventual bronchiectasis. The resultant morbidity and mortality rates from pulmonary disease approach 95%; with lung transplantation remaining the only definitive cure for CF. Therefore, pulmonary imaging plays a vital role in the early detection, longitudinal monitoring, and pre-transplant and post-transplant evaluation of patients with CF.
This article has the two following purposes: (1) to review the specific imaging features of CF with conventionally used imaging modalities (chest radiographs and high-resolution computed tomography [HRCT]) as well as with emerging imaging technologies (digital chest tomosynthesis, hyperpolarized gas magnetic resonance (MR) imaging, and noncontrast MR imaging); and (2) to review the HRCT imaging findings that are essential in the evaluation of these patients in the pre–lung transplant and post–lung transplant settings.
Imaging techniques
Conventional Imaging Techniques
Chest radiographs
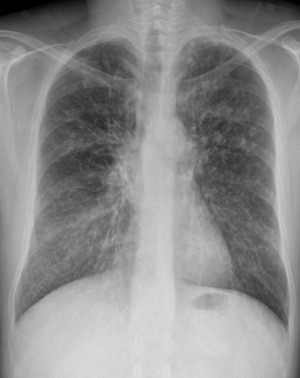
Chest radiographs are the most commonly used and often the initial imaging modality when evaluating patients with CF in both pediatric and adult populations. Although less sensitive than HRCT, chest radiographs continue to be used because of the low radiation dose, low cost, and widespread availability. Early in the disease process, chest radiographs may appear entirely normal. The earliest radiographic findings include hyperinflation secondary to distal airway mucus obstruction or cylindrical bronchiectasis (with tram track opacities). , By the time patients with classic CF reach adulthood, chronic episodes of mucus obstruction and bronchopulmonary infection result in classic chest radiograph findings, including central and upper lung predominant varicoid and cystic bronchiectasis, bronchial wall thickening, peribronchial cuffing, increased interstitial markings, and scattered nodular opacities ( Fig. 1 ). , Pneumothoraces are frequently seen and can be recurrent. , , Patients with nonclassic CF tend to be adults with fewer clinical symptoms and can have chest radiographs demonstrating mild to very subtle or absent radiographic changes when compared with those of patients with classic CF.
Superimposed bacterial infection (such as with Pseudomonas aeruginosa or Burkholderia species) often results in mucoid impaction or areas of consolidation ( Fig. 2 ). Mucus-impacted central bronchiectasis in a so-called finger-in-glove appearance is classically observed in the setting of allergic bronchopulmonary aspergillosis (ABPA) and develops in up to 20% of patients with CF. ,

Several chest radiograph scoring systems for CF exist, including the Brasfield, Chrispin-Norman, and Wisconsin systems. Most of these involve scoring of air trapping, bronchiectasis, disease extent, and severity, and all of these have been demonstrated to have good correlation with lung function. Ultimately, choosing which scoring system to evaluate patients with CF for longitudinal monitoring is the responsibility of the reader or institution. Recently developed machine learning models using deep convolution neural networks have been used in the scoring of chest radiographs with one study reporting similar accuracy of a machine learning algorithm to a pediatric radiologist in predicting the Brasfield score. Although promising, these models remain in infancy, and their exact clinical role has yet to be determined.
High-resolution computed tomography
HRCT remains the current gold standard in the evaluation of CF with several studies demonstrating superior accuracy when compared with incentive spirometry and chest radiography alone. HRCT findings of classic CF include central and upper lung predominant varicoid and cystic bronchiectasis, bronchial wall thickening, endobronchial mucoid impaction, hyperinflation with air trapping, distal atelectasis, and sacculations ( Fig. 3 ).

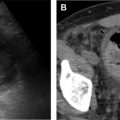
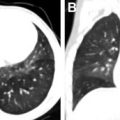
Superimposed infection can have a variety of imaging manifestations. In the setting of bacterial infection (commonly Pseudomonas ), lobar and multifocal consolidation, abscesses, and cavities can be observed ( Fig. 4 ). In the setting of ABPA, mucus accumulation within an impacted bronchiectactic airway results in a mucocele. High attenuation of mucus is observed in up to 25% of patients and is the result of chelated calcium, iron, and manganese , ( Fig. 5 ). Approximately 10% of adult patients with CF are infected with atypical nontuberculous mycobacteria (NTM), frequently Mycobacterium avium complex and Mycobacterium abscessus , with classic HRCT findings, including centrilobular and tree-in-bud micronodules, which can wax and wane following episodes of treatment ( Fig. 6 ). ,



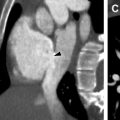
In advanced disease, repeat episodes of inflammation and long-standing bronchiectasis result in angiogenesis and hypertrophied bronchial arteries that may subsequently cause massive hemoptysis, which is observed in up to 4% of patients with CF. Many patients present to the emergency department with hemoptysis and undergo a computed tomographic (CT) angiogram to identify the source of bleeding. CT angiography may demonstrate “filling defects” and flow artifacts, which can mimic acute or chronic emboli. However, careful attention to the course of adjacent bronchial collaterals as well as performing a slightly delayed scan (by bolus triggering with a tracker placed over the descending thoracic aorta instead of the main pulmonary artery) may help elucidate the true nature of these “smoke-like filling defects” ( Fig. 7 ). Because bronchial artery embolization is the treatment of choice in such scenarios, it is paramount that hypertrophied bronchial arteries be reported in these patients to best provide a vascular roadmap for interventionalists. Additional HRCT findings of CF include mediastinal and hilar lymphadenopathy, secondary to reactive inflammation, and pneumothoraces ( Figs. 8 and 9 ). ,



Although several HRCT scoring systems have been reported, there is currently no consensus scoring system identified. , In particular, the Bhalla scoring system examines the severity and extent of bronchiectasis, peribronchial thickening, extent of mucus plugging, emphysema, as well as the presence of abscesses, bubbles, and sacculations. Studies have reported good correlation with the Bhalla score and pulmonary function, as determined by incentive spirometry, as well as that an increased Bhalla score had a high correlation with Pseudomonas infection. However, it is ultimately the reader’s responsibility in choosing a scoring system to use if using one at all.
Recently, several automated and semiautomated HRCT quantification methods have been designed to evaluate the severity of patients with CF and to monitor longitudinal progression. However, these technologies are still in their infancy, and further studies will need to be performed to fully understand their roles in the future.
Emerging Imaging Techniques
Digital chest tomosynthesis
New technical developments in digital chest tomosynthesis have led to its emergence as a potential alternative imaging modality in the evaluation of CF. Chest tomosynthesis images are generated from several low-dose exposures obtained during breath-hold by a moving radiograph tube over a short arc. Images are then reconstructed into a tomographic composite image consisting of up to 60 coronal images with a slice thickness of 3 mm in children and 4 mm in adults. Compared with HRCT, tomosynthesis allows for higher spatial resolution with a comparatively lower radiation dose (0.1 to 0.2 msV vs 4 to 8 msV). ,
Tomosynthesis findings of CF are similar to that of conventional chest radiographs and include hyperinflation, bronchiectasis, peribronchial thickening, mucus plugging, and consolidation. However, unlike conventional radiographs, tomosynthesis allows for superior evaluation of the airways and vascular tree. Although central bronchiectasis is present on both radiographs and tomosynthesis, peripheral bronchiectasis can be more easily detected on tomosynthesis studies. Classification of bronchiectasis (cylindrical, varicoid, or cystic) can be easily characterized with tomosynthesis compared with chest radiographs. Areas of endobronchial mucus impaction and clustered nodules are more readily evident on tomosynthesis and may only appear as vague or blurred shadows on radiography ( Fig. 10 ). Infectious complications, such as abscesses and consolidation, and noninfectious complications, such as bulla and pneumothoraces, are also more easily detectable on tomosynthesis and may be missed on conventional radiographs.

Currently, two scoring systems exist for digital chest tomographic evaluation of CF. Vult von Steyern and colleagues developed a scoring system incorporating increased lung volumes, bronchial wall thickening, bronchestasis, mucus plugging, and atelectasis/consolidation and reported good correlation with radiographs scored with the Brasfield scoring system. A second scoring system developed by Gunnell and colleagues demonstrated superior correlation to forced expiratory volume in one second (FEV 1 ) when compared with Brasfield scored chest radiographs.
Several technical limitations of tomosynthesis are present when compared with HRCT and include decreased depth resolution, susceptibility to image degradation by artifact from support device hardware, longer imaging times, requirement for anesthesia in younger patients, and inability to construct multiplanar reformatted images. In particular, in severely dyspneic patients, the breath-hold of 10 seconds required for tomosynthesis may not be easily achievable. In addition, early findings of CF, such as areas of air trapping manifesting as mosacism on HRCT, may be occult on tomosynthesis, leading to a delayed diagnosis. Despite these limitations, tomosynthesis offers superior evaluation for CF when compared with conventional radiography with a comparatively lower radiation dose increase between chest radiographs (0.01 mSv) and tomosynthesis (0.1–0.2 msV) when compared with HRCT (4–8 msV). , A potential emerging role for tomosynthesis in the evaluation of patients with CF might be as an intermediate imaging modality to further characterize abnormalities detected on surveillance chest radiographs in such scenarios whereby the detail of HRCT is not required as a means to mitigate radiation dose.
Magnetic resonance imaging
A major limitation of chest radiographs, HRCT, and digital chest tomosynthesis is its use of ionizing radiation, especially for longitudinal monitoring because several repeat studies are necessary to monitor CF disease progression and treatment over several years and decades. MR imaging avoids this limitation with several imaging techniques recently introduced to evaluate pulmonary function, including (1) hyperpolarized gas MR imaging and (2) noncontrast MR imaging techniques.
Hyperpolarized gas MR imaging
First reported in 1999, ventilation MR imaging using hyperpolarized gases (either helium-3 [He-3] or xenon-129 [Xe-129]) has been investigated as an alternative imaging modality to HRCT in the evaluation of CF. , He-3 was the first reported hyperpolarized ventilation gas agent with MR imaging performed using a breath-hold technique following administration. By quantifying the region of lung containing low or absent signal (the ventilation defect) and comparing it to the total lung area, a ventilation defect percentage can be obtained ( Fig. 11 ). Previous studies have demonstrated that the ventilation defect percentage has good correlation with the degree of pulmonary dysfunction as determined by incentive spirometry and can be used to accurately assess early disease progression or treatment response. , Disadvantages of He-3 gas MR include the necessity for on-site production of hyperpolarized gas, scarcity of He-3, breath-hold technique in particularly dyspneic patients, and the comparatively low spatial resolution compared with HRCT.