Chapter 9).
CT is the best imaging modality for radiotherapy in terms of dosimetry and spatial accuracy, but does not always provide the best images for tumour or critical structure localization. Normal practice is to register the diagnostically superior magnetic resonance (MR) or positron emission tomography (PET) images onto the CT images (see
When using a TPS with 3D scatter correction, it is important that the CT scan extends beyond the PTV, ideally by at least 2 cm, so that the adjacent anatomy is accounted for in the calculation and not assumed to be air by the TPS.
The contours used for planning must represent the patient contour during treatment delivery – bearing in mind that treatment will take several minutes, whereas CT scans are acquired in seconds. Most contouring techniques, whether CT or external outlines only, are very rapid ‘snapshots’ compared to treatment delivery times. Therefore, either the planning procedure, or the treatment delivery, must account for patient movement. For the vast majority of treatments, the treatment plan must be designed to account for this patient movement by the addition of margins around the clinical target volume. However, modern technology now offers practical methods of ‘gated’ treatments that only deliver the dose to the patient while they are in the same position as during the therapy CT scan, or ‘tracking’ treatments, that can follow the PTV movement. These are discussed in the final section of this chapter.
 shows the effect of patient breathing on a thoracic CT image acquired in spiral mode. The appearance of the liver clearly indicates the breathing motion that otherwise might not be apparent.
shows the effect of patient breathing on a thoracic CT image acquired in spiral mode. The appearance of the liver clearly indicates the breathing motion that otherwise might not be apparent.
Radiotherapy patients may have prostheses, dental fillings, breast implants, and other artifacts present externally or internally. Sometimes these will be present during imaging but may be removed by the time treatment commences (for example, drainage tubes). Where the artifact is made of material similar to tissue density such as breast implants (although some breast implants contain high-density magnets), the CT-density table will correctly allocate the density required. However, for metal implants, the CT scan must be acquired with an extended CT scale and the TPS CT-density table must be extended typically to give Hounsfield numbers of up to 32 000 if metal implants are to be identified and the correct density assigned. Even with artifact correction tools on CT scanners, there are usually significant artifacts present surrounding metal objects – especially for bilateral hip implants, and a manual overlay of the density may be required (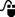 ). The use of MR imaging in the presence of non-ferrous implants may provide useful information that may be transferred to the distorted CT dataset.
). The use of MR imaging in the presence of non-ferrous implants may provide useful information that may be transferred to the distorted CT dataset.
Cardiac pacemakers present a problem, not only in perturbing the treatment beam, but also in their sensitivity to radiation and electromagnetic interference from linear accelerators. Ideally, pacemakers should not lie in the path of a treatment field and should only receive scattered radiation dose of less than 2 Gy. The cardiologist should be consulted to ascertain the required level of monitoring during and after the treatment.
Contrast may be present in a CT scan, for example, to visualize nodes in head and neck images ( ), or may be present in the bladder as a result of contrast used elsewhere.
), or may be present in the bladder as a result of contrast used elsewhere.
This contrast will not usually be present during the actual treatment, but its presence during planning does not usually have a significant impact on the dosimetric accuracy. However, this should be verified whenever a new contrast-based localization technique is introduced.
Care should be taken to ensure that the CT image represents the treatment set-up. Sometimes, the patient cannot be scanned in the same position as for treatment due to restrictions of the CT scanner aperture, particularly when immobilization devices are employed. For some sites, the scan may be performed feet first into the scanner while the patient may be treated in the head first orientation. In this case, the TPS or scanner software may be utilized to mirror and restack the scans into the treatment position. When such techniques are employed, there must be clearly visible quality assurance (QA) tools to verify that the software has processed the images correctly, such as couch markers identifying the couch superior-inferior and lateral orientations.
The CT couch, immobilization supports, bolus, or non-treatment-related devices may be present in the CT scan. The user should be aware of how the TPS will handle, or ignore, structures that lie outside the patient’s external contour and how to compensate for TPS calculations that do not account for beams passing through these structures. High density couch bars on the treatment unit couch may need to be accounted for in plans with beams passing through the couch and, ideally, the positions of these bars should be overlaid on the CT image so that the plan is designed to avoid them.
The planner should also be aware of any anatomical abnormalities in the CT scan that may affect the treatment plan. It may be preferable to avoid regions where surgery has taken place, or to avoid abnormalities unrelated to the disease being treated, such as a hernia.
It is important to appreciate the shortcomings of the TPS algorithms, especially in the presence of heterogeneities. The build-up effect at internal density boundaries may not be accurate and the 3D scatter distribution may not be accurately modelled. These effects typically lead to the TPS overestimating doses in soft tissue regions (e.g. tumours) surrounded by low density tissue (e.g. lung).
For simple point dose calculations, a water phantom can be used to approximate the patient anatomy. This may be performed manually using tabulated data direct from plotting tank measurements, or by a TPS assuming the patient to be an infinite water phantom. In either case, the actual patient contour may differ significantly from the phantom and where the point dose lies near the patient surface or internal heterogeneities, discrepancies of several percent may exist if comparing against calculations performed with full CT data.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree