(1)
Department of Nuclear Medicine, INHS, Asvini, Mumbai, India
Abstract
The term electromagnetic radiation or electromagnetic waves refers to energy in the form of oscillating electric and magnetic fields. Individual packets of electromagnetic radiation are referred to as photons. Photons with energy greater than 100 eV are classified as X-rays or gamma rays. Lower-energy photons may be in the range of ultraviolet light, infrared, visible light, radar waves, or radio and television waves. The unit of energy used to describe these electromagnetic waves or radiations is the electron volt (eV). One electron volt is defined as the kinetic energy of an electron accelerated through a potential difference of 1 V (1 eV = 1.6 × 10−19 J or 1.6 × 10−12 erg).
5.1 Electromagnetic Radiation and Radioactivity
5.1.1 Electromagnetic Radiation
The term electromagnetic radiation or electromagnetic waves refers to energy in the form of oscillating electric and magnetic fields. Individual packets of electromagnetic radiation are referred to as photons. Photons with energy greater than 100 eV are classified as X-rays or gamma rays. Lower-energy photons may be in the range of ultraviolet light, infrared, visible light, radar waves, or radio and television waves. The unit of energy used to describe these electromagnetic waves or radiations is the electron volt (eV). One electron volt is defined as the kinetic energy of an electron accelerated through a potential difference of 1 V (1 eV = 1.6 × 10−19 J or 1.6 × 10−12 erg).
5.1.2 Radioactivity and Radioactive Materials
There are about 2,450 known isotopes of the 100 odd elements in the periodic table. The unstable isotopes lie above or below the nuclear stability curve (a graph of the number of protons against the number of neutrons). These unstable isotopes attempt to reach the stability curve by splitting into fragments, in a process called fission, or by emitting particles and/or energy in the form of radiation. This latter process is called radioactivity. The materials which are radioactive in nature are called radioactive material.
Radioactivity was discovered in 1896 by the French scientist Henri Becquerel, while working on phosphorescent materials [1].
5.1.2.1 Units of Radioactivity Measurements
The SI unit of radioactive activity is the becquerel (Bq). Earlier there was another unit of radioactivity called curie (Ci). 1 curie (Ci) = 3.7 × 1010 Bq.
5.1.3 Radioisotopes
Unstable atoms, for instance, those that have too many protons to remain a stable entity, are called radioactive isotopes or radioisotopes or radionuclide.
About 300 of the 2,450-odd isotopes are found in nature. The rest are man-made, that is, they are produced artificially.
5.1.4 Generators
A generator is a device containing a long-lived parent and short-lived daughter in a state of radioactive equilibrium. The device enables repeated separation, adopting simple means of the short-lived daughter in as pure nuclear form as possible throughout the operating life of the generator. Examples are 99Mo–99mTc generators and 81Rb–81mKr generators.
5.1.5 Half-Life
Half-life expresses the length of time it takes for the radioactivity of a radioisotope to decrease by a factor of 2. Half-life does not express how long a material will remain radioactive but simply the length of time for its radioactivity to halve. Examples of some of the radioisotopes used in nuclear medicine:
Radioisotope | Half-life (approx.) |
|---|---|
11C (carbon) | 20.4 min |
14C (carbon) | 5,730 years |
13 N (nitrogen) | 10 min |
15O (oxygen) | 122 s |
18 F (fluorine) | 110 min |
32P (phosphorus) | 14.29 days |
51Cr (chromium) | 1 month |
57Co (cobalt) | 270.9 days |
67Ga (gallium) | 78.26 h |
68Ga (gallium) | 68 min |
81mKr (krypton) | 13 s |
82 Rb (rubidium) | 1.3 min |
90Y (yttrium) | 64 h |
99Mo (molybdenum) | 66 h |
99mTc (technetium) | 6.02 h |
111In (indium) | 2.83 days |
123I (iodine) | 13.2 h |
125I (iodine) | 60 days |
131I (iodine) | 8.04 days |
133Xe (xenon) | 5.25 days |
137Cs (cesium) | 30 years |
201Tl (thallium) | 3.04 days |
5.1.6 Radiopharmaceutical
Radiopharmaceuticals are a combination of a radioactive molecule (permits external detection) with certain compound which is a biologically active molecule or drug that acts as a carrier and determines localization and biodistribution. Radiopharmaceuticals portray the physiology, biochemistry, or pathology of a body system without causing any perturbation of function.
They are also referred to as a radiotracer because they are given in subpharmacological doses that “trace” a particular physiological and pathological process in the body.
5.2 Radiation Safety
5.2.1 Energy
Energy indicates the quality (penetrating power) of radiation.
Conventionally electron volts (eV) and its multiples are used for expressing the energy of radiation.
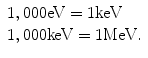
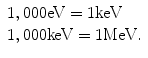
5.2.2 Exposure (X)
It quantifies the amount of indirect ionizing radiation (photons) present at any point in air. It is the amount of x- or γ-radiation which produces ionization of one electrostatic unit (esu) of either positive or negative charge per cubic cm of dry air at standard temperature and pressure (STP), i.e., at 0° temperature and 760 mm of pressure.
SI unit: coulomb per kg (C/kg)
Old unit: roentgen (R)
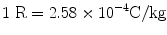
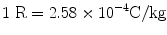
5.2.3 Exposure Rate (X˚)
Exposure per unit time is called exposure rate.
SI unit: coulomb/kg.sec or ampere/kg
Old unit: roentgen per hour
5.2.4 Dose (D)
It is a measure of amount of energy absorbed per unit mass of the matter by any type of radiation.
SI unit: joule per kg (J/kg) or gray (Gy)
Old unit: rad (radiation absorbed dose)
1 Gy = 100 rad, 1 rad = 100 ergs/g
5.2.5 Dose Rate (D˚)
Dose per unit time is called dose rate.
SI unit: joule per kg (J/kg) or gray (Gy)/h
Old unit: rad (radiation absorbed dose)/h
5.2.6 Relationship Between Roentgen and Rad
In tissue:
1 R ≈ 1 Rad
or 1 R ≈ 10 mGy
5.2.7 Equivalent Dose (H)
It accounts for the differences in effectiveness of different types of radiation causing biological damage:
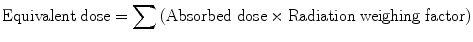
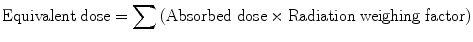
Or H = ∑ (D × W R)
where W R is radiation weighting factor or quality factor. It is different for different types of radiation. However, W R is 1 for α, β, and γ. Therefore, for use in nuclear medicine,
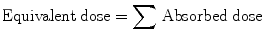
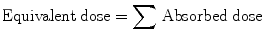
Unit: SI unit is sievert (SV) or rem (roentgen equivalent in man).
1 SV = 100 rem.
5.2.8 Effective Dose (E)
Different organs have different radiosensitivity. Therefore, they receive different doses. To calculate that effective dose term is used:
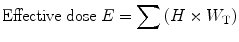 where W T is tissue weighting factor.
where W T is tissue weighting factor.
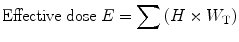
Unit: sievert (SV)
Tissue weighting factor of different organs (as per ICRP-103 published in year 2007):
Gonads | 0.08 |
Breast | 0.12 |
Bone marrow | 0.12 |
Lung | 0.12 |
Liver | 0.04 |
Stomach | 0.12 |
Colon | 0.12 |
Thyroid | 0.04 |
Esophagus | 0.04 |
Bladder | 0.04 |
Skin | 0.01 |
Bone surface | 0.01 |
Brain | 0.01 |
Salivary gland | 0.01 |
Remaindera | 0.12 |
5.2.9 Cumulative Dose
It is considered for an occupational worker for the period of his life span. It is considered 50 year.
Unit: sievert (SV)
5.2.10 Collective Dose
It is the amount of exposure in radiation field received by personnel.
Unit: person-sievert (SV)
5.2.11 Annual Limit on Intake (ALI)
It is the amount of permissible limit of radiation in the environment of radiation field where occupational worker works. It is different for different radionuclides.
One ALI
For 131I = 01 MBq
99mTc = 2,000 MBq
125I = 02 MBq
One ALI should not cross in a year.
5.2.12 Derived Air Concentration (DAC)
It is the amount of activity per cubic meter in a radiation field. It is derived from ALI.
For occupational worker:
No. of hours working in a year (assuming 8 h in a day, 5 days in week, and 50 weeks in a year) = 8 × 5 × 50 = 2,000 h.
Breathing air by a person = 1.2 m3/h
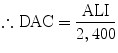
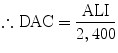
e.g., for 131I
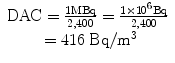
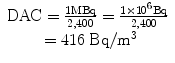
Similarly,
for 125I = 830 Bq/m3
99mTc = 8.3 × 105 Bq/m3
18 F = 3.8 × 105 Bq/m3
Limit of radiation field:
For occupational worker, 1 mR/h (1 mR = 10 μSv & 1 Sv = 100 R)
For public, 0.1 mR/h or 1 μSv
5.2.13 Limits of Contamination
Different areas
General corridor – 10−5 μCi or 0.37 Bq/cm2
Work bench or lab – 10−4 μCi or 3.7 Bq/cm2
Fume hood – 10−3 μCi or 37 Bq/cm2
For body
Skin – 1.5 Bq/cm2
Hands – 350 Bq/cm2
Clothes – 02 Bq/cm2
Shoes – 0.37 Bq/cm2
5.2.14 Half-Value Thickness or Layer (HVT or HVL)
The thickness of media, which reduces radiation intensity to half of its initial intensity, is known as half-value thickness or half-value layer. HVT depends upon:
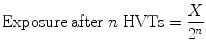 where X = exposure
where X = exposure
1.
Energy of radiation
2.
Density of medium
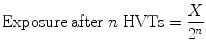
5.2.15 Tenth Value Thickness or Layer (TVT or TVL)
The thickness of attenuating medium which reduces the radiation intensity to 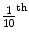 of its incident intensity is known as tenth value thickness or tenth value layer.
of its incident intensity is known as tenth value thickness or tenth value layer.
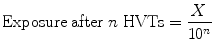
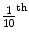 of its incident intensity is known as tenth value thickness or tenth value layer.
of its incident intensity is known as tenth value thickness or tenth value layer.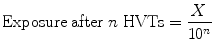
5.2.16 Relationship Between HVT and TVT
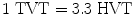
5.2.17 Exposure Rate Constant
It represents exposure rate from 1 mCi point source at a distance of 1 cm.
Radioisotope | Exposure rate constant (in R/h) | Half-value thickness of lead (in cm) |
|---|---|---|
99mTc | 0.8 | 0.03 |
99Mo | 1.46 | 0.7 |
131I | 2.2 | 0.24 |
125I | 1.4 | 0.002 |
18 F | 5.7 | 0.39 |
57Co | 0.56 | 0.02 |
201Tl | 0.45 | 0.02 |
67Ga | 0.76 | 0.10 |
123I | 1.55 | 0.04 |
111In | 2.05 | 0.10 |
5.2.18 Discharge Criteria for Patient (as per Atomic Energy Regulatory Board, India)
At 1 m distance:
>30 mCi or 1,110 MBq or 5 mR/h
Isolation
<30 mCi or 1,110 MBq or 5 mR/h
Discharge/OPD
∴ If there is exposure rate of less than 5 mR/h at 1 m distance, the patient is discharged.
5.2.19 Dose Limits Recommended by ICRP (2007)
Exposure condition | Dose limit (mSv per year) | ||
|---|---|---|---|
Occupational | Apprentices | Public | |
Whole body (effective dose) | 20 mSv per year averaged over defined period of 5 years with no more than 50 mSv in a single year | 6 mSv in a year | 1 mSv in a year averaged over 5 years |
Parts of the body (equivalent dose) | |||
Lens of the eye | 150 mSv per year | 50 mSv in a year | 15 mSv in a year |
Skina | 500 mSv per year | 150 mSv in a year | 50 mSv in a year |
Hands and feetb | 500 mSv per year | 150 mSv in a year | 50 mSv in a year |
5.3 Endocrine System
5.3.1 Graves’ Disease (Diffuse Toxic Goiter)
Graves’ disease is an autoimmune disease where the thyroid is overactive, producing an excessive amount of thyroid hormones (a serious metabolic imbalance known as hyperthyroidism and thyrotoxicosis). This is caused by thyroid autoantibodies that activate the TSH receptor, thereby stimulating thyroid hormone synthesis and secretion and thyroid growth (causing a diffusely enlarged goiter). The resulting state of hyperthyroidism can cause a dramatic constellation of neuropsychological and physical signs and symptoms [2].
Graves’ disease is the most common cause of hyperthyroidism and usually presents itself during midlife, but also appears in children, adolescents, and the elderly. Graves’ disease is also the most common cause of severe hyperthyroidism, which is accompanied by more clinical signs and symptoms and laboratory abnormalities as compared with milder forms of hyperthyroidism [3]. About 30–50 % of people with Graves’ disease will also suffer from Graves’ ophthalmopathy (a protrusion of one or both eyes), caused by inflammation of the eye muscles by attacking autoantibodies.
Diagnosis is usually made on the basis of symptoms and thyroid hormone tests [4]. Graves’ thyrotoxicosis frequently builds over an extended period, sometimes years, before being diagnosed [5]. This is partially because symptoms can develop so insidiously that they go unnoticed; when they do get reported, they are often confused with other health problems. Thus, diagnosing thyroid disease clinically can be challenging [6]. Nevertheless, patients can experience a wide range of symptoms and suffer major impairment in most areas of health-related quality of life [7].
Graves’ disease has no cure, but treatments for its consequences (hyperthyroidism, ophthalmopathy, and mental symptoms) are available [8]. The Graves’ disease itself—as defined, for example, by high serum thyroid autoantibodies (TSHR-Ab) concentrations or ophthalmopathy—often persists after its hyperthyroidism has been successfully treated [6].
5.3.2 Plummer’s Disease (Toxic Multinodular Goiter)
Plummer’s disease is named after the American physician Henry Stanley Plummer [9] but refers to a single toxic nodule (adenoma) which may present with the background of a suppressed multinodular goiter.
Toxic multinodular goiter (also known as toxic nodular goiter, toxic nodular struma) is a form of hyperthyroidism—where there is excess production of thyroid hormones. It is characterized by functionally autonomous nodules. It emerges insidiously from nontoxic multinodular goiter.
It is the second most common cause of hyperthyroidism (after Graves’ disease) in the developed world. In countries where the population is iodine deficient, i.e., the developing world, iodine deficiency is the most common cause of hypothyroidism. (Decreased iodine leads to decreased thyroid hormone.) However, iodine deficiency can cause goiter (thyroid enlargement); within a goiter, nodules can develop.
Symptoms of toxic multinodular goiter are similar to that of hyperthyroidism, including
Heat intolerance
Muscle weakness/wasting
Hyperkinesis
Tremor
Irritability
Weight loss
Osteoporosis
Increased appetite
Goiter (swelling of the thyroid gland)
Tachycardia (high heart rate, above 100 bpm at rest in adults)
Causes
Sequence of events [10]:
1.
Iodine deficiency leading to decreased T4 production.
2.
Induction of thyroid cell hyperplasia due to low levels of T4. This accounts for the multinodular goiter appearance.
3.
Increased replication predisposes to a risk of mutation in the TSH receptor.
4.
If the mutated TSH receptor is constitutively active, it would then become “toxic” and produces excess T3/T4 leading to hyperthyroidism.
5.3.3 Radioiodine Therapy
Iodine has a property to be trapped by the thyroid gland. Radioactive iodine is used to destroy malfunctioning thyroid tissues because of its beta and gamma emission. There is over 50 years of experience with the use of therapeutic I-131 [11]. It has high efficacy and low incidence of adverse effects [11]. Common indications of radioiodine therapy are Graves’ disease, Plummer’s disease, and functioning thyroid cancer.
5.3.4 Toxic Multinodular Goiter
Toxic multinodular goitre is a form of hyperthyroidism – where there is excess production of thyroid hormones. It is characterized by functionally autonomous nodules. It emerges insidiously from nontoxic multinodular goitre.
It is the second most common cause of hyperthyroidism (after Graves’ disease) in the developed world. In countries where the population is iodine-deficient i.e. the developing world, iodine deficiency is the most common cause of hypothyroidism. (Decreased iodine leads to decreased thyroid hormone.) However, iodine deficiency can cause goitre (thyroid enlargement); within goitre, nodules can develop.
Symptoms are heat intolerance, muscle weakness/wasting, hyperkinesis, tremor, irritability, weight loss, osteoporosis, increased appetite, goitre (swelling of the thyroid gland), tachycardia (high heart rate – above 100 bpm at rest in adults).
Sequence of events
1.
Induction of thyroid cell hyperplasia due to low levels of T4. This leads to multinodular goitre appearance.
2.
Increased replication predisposes to a risk of mutation in the TSH receptor.
3.
If the mutated TSH receptor is constitutively active, it would then become ‘toxic’ and produces excess T3/T4 leading to hyperthyroidism.
5.3.5 Thyroiditis
Thyroiditis is the inflammation of the thyroid gland. The thyroid gland is located on the front side the neck below the laryngeal prominence and makes hormones that control metabolism.
Thyroiditis is a group of disorders that all cause thyroidal inflammation. Forms of the disease are Hashimoto’s thyroiditis, the most common cause of hypothyroidism in the USA; postpartum thyroiditis; subacute thyroiditis; silent thyroiditis; drug-induced thyroiditis; radiation-induced thyroiditis; acute thyroiditis; and Riedel’s thyroiditis [12].
Each different type of this disease has its own causes, clinical features, diagnoses, durations, resolutions, conditions, and risks.
Common symptoms are the same as in hypothyroid symptoms manifest when thyroid cell damage is slow and chronic and may include fatigue, weight gain, feeling “fuzzy headed,” depression, dry skin, and constipation. Other rarer symptoms include swelling of the legs, vague aches and pains, decreased concentration, and so on. When conditions become more severe, depending on the type of thyroiditis, one may start to see puffiness around the eyes, slowing of the heart rate, a drop in body temperature, or even incipient heart failure. On the other hand, if the thyroid cell damage is acute, the thyroid hormone within the gland leaks out into the bloodstream causing symptoms of thyrotoxicosis, which is similar to those of hyperthyroidism. These symptoms include weight loss, irritability, anxiety, insomnia, fast heart rate, and fatigue. Elevated levels of thyroid hormone in the bloodstream cause both conditions, but thyrotoxicosis is the term used with thyroiditis since the thyroid gland is not overactive, as in the case of hyperthyroidism [13, 14].
Treatments for this disease depend on the type of thyroiditis that is diagnosed. For the most common type, which is known as Hashimoto’s thyroiditis, the treatment is to start hormone replacement if hypothyroidism develops. This prevents or corrects the hypothyroidism, and it also generally keeps the gland from getting bigger [15]. Often, victims of this disease only need bed rest and nonsteroidal anti-inflammatory medications; however, some need steroids to reduce inflammation and to control palpitations. Depending on the type of thyroiditis, doctors may prescribe drugs called beta-blockers to lower the heart rate and reduce tremors [16].
5.3.6 Hashimoto’s Thyroiditis
Hashimoto’s thyroiditis was first described by the Japanese physician Hashimoto Hakaru working in Germany in 1912. Hashimoto’s thyroiditis is also known as lymphocytic thyroiditis, and patients with this disease often complain about difficulty in swallowing. This condition may be so mild at first that the disease goes unnoticed for years. The first symptom that shows signs of Hashimoto’s thyroiditis is goiter on the front of the neck [15]. Depending on the severity of the disease and how much it has progressed, doctors then decide what steps are taken for treatment.
5.4 Skeletal System
5.4.1 Arthropathy
An arthropathy is a disease of a joint. Arthritis is a form of arthropathy that involves inflammation of one or more joints [17], while the term arthropathy may be used regardless of whether there is inflammation or not.
Spondyloarthropathy is any form of arthropathy of the vertebral column [18].
Arthropathy may also include joint conditions caused by physical trauma to joints, but is traditionally used to describe the following conditions:
Reactive arthropathy is caused by an infection, but not a direct infection of the synovial space.
Enteropathic arthropathy is caused by colitis and related conditions.
Crystal arthropathy (also known as crystal arthritis) involves the deposition of crystals in the joint.
Diabetic arthropathy is caused by diabetes.
Neuropathic arthropathy is associated with a loss of sensation.
5.4.2 Avascular Necrosis
Avascular necrosis (AVN) is a disease where there is cellular death (necrosis) of bone components due to interruption of the blood supply [19]. Without blood, the bone tissue dies and the bone collapses. If avascular necrosis involves the bones of a joint, it often leads to destruction of the joint articular surfaces.
There are many theories about what causes avascular necrosis. Proposed risk factors include chemotherapy, alcoholism [20], excessive steroid use [21], post-trauma [22, 23], caisson disease (decompression sickness) [24, 25], vascular compression [26], hypertension, vasculitis, arterial embolism and thrombosis, damage from radiation, bisphosphonates (particularly the mandible) [27], sickle cell anemia [28], and deep diving [29].
In some cases it is idiopathic (no cause is found) [30]. Rheumatoid arthritis and lupus are also common causes of AVN. Prolonged, repeated exposure to high pressures (as experienced by commercial and military divers) has been linked to AVN, though the relationship is not well understood.
Avascular necrosis is especially common in the hip joint. A variety of methods are now used to treat avascular necrosis, the most common being the total hip replacement, or THR. However, THRs have a number of downsides including long recovery times and short life spans.
5.4.3 Hypertrophy
Hypertrophy is the increase in the volume of an organ or tissue due to the enlargement of its component cells. It should be distinguished from hyperplasia, in which the cells remain approximately the same size but increase in number. Although hypertrophy and hyperplasia are two distinct processes, they frequently occur together.
5.4.4 Leukemia
Leukemia is a type of cancer of the blood or bone marrow characterized by an abnormal increase of immature white blood cells called “blasts.” Leukemia is a broad term covering a spectrum of diseases. In turn, it is part of the even broader group of diseases affecting the blood, bone marrow, and lymphoid system, which are all known as hematological neoplasm.
In 2000, approximately 256,000 children and adults around the world developed some form of leukemia, and 209,000 died from it [31].
Diagnosis is usually based on repeated complete blood counts and a bone marrow examination following observations of the symptoms; however, in rare cases, blood tests may not show if a patient has leukemia, usually this is because the leukemia is in the early stages or has entered remission. A lymph node biopsy can be performed as well in order to diagnose certain types of leukemia in certain situations.
Most forms of leukemia are treated with pharmaceutical medication, typically combined into a multidrug chemotherapy regimen. Some are also treated with radiation therapy. In some cases, a bone marrow transplant is useful.
5.4.5 Lymphoma
Lymphoma is a cancer of the lymphocytes, a type of cell that forms part of the immune system. Typically, lymphoma is present as a solid tumor of lymphoid cells. Treatment might involve chemotherapy and in some cases radiotherapy and/or bone marrow transplantation and can be curable depending on the histology, type, and stage of the disease [32]. These malignant cells often originate in lymph nodes, presenting as an enlargement of the node (a tumor). It can also affect other organs in which case it is referred to as extranodal lymphoma. Extranodal sites include the skin, brain, bowels, and bone. Lymphomas are closely related to lymphoid leukemias, which also originate in lymphocytes but typically involve only the circulating blood and bone marrow (where blood cells are generated in a process termed hematopoiesis) and do not usually form static tumors [32]. There are many types of lymphomas, and in turn, lymphomas are a part of the broad group of diseases called hematological neoplasm.
Thomas Hodgkin published the first description of lymphoma in 1832, specifically of the form named after him, Hodgkin’s lymphoma [33]. Since then, many other forms of lymphoma have been described, grouped under several proposed classifications. The 1982 Working Formulation classification became very popular. It introduced the category non-Hodgkin’s lymphoma (NHL), divided into 16 different diseases. However, because these different lymphomas have little in common with each other, the NHL label is of limited usefulness for doctors or patients and is slowly being abandoned. The latest classification by the WHO (2008) lists 70 different forms of lymphoma divided in four broad groups [34].
5.4.6 Multiple Myeloma
Multiple myeloma, also known as plasma cell myeloma or Kahler’s disease (after Otto Kahler), is a cancer of plasma cells, a type of white blood cell normally responsible for producing antibodies [35]. In multiple myeloma, collections of abnormal plasma cells accumulate in the bone marrow, where they interfere with the production of normal blood cells. Most cases of myeloma also feature the production of a paraprotein—an abnormal antibody which can cause kidney problems. Bone lesions and hypocalcemia (high calcium levels) are also often encountered [35].
Myeloma is diagnosed with blood tests (serum protein electrophoresis, serum free kappa/lambda light chain assay), bone marrow examination, urine protein electrophoresis, and X-rays of commonly involved bones. Myeloma is generally thought to be treatable but incurable.
Myeloma develops in 1–4 per 100,000 people per year. It is more common in men and for unknown reasons is twice as common in African Americans as it is in white Americans. With conventional treatment, median survival is 3–4 years, which may be extended to 5–7 years or longer with advanced treatments. Multiple myeloma is the second most common hematological malignancy in the USA (after non-Hodgkin’s lymphoma) and constitutes 1 % of all cancers [35].
5.4.7 Metastasis
Metastasis or metastatic disease or mets is the spread of a disease from one organ or part to another nonadjacent organ or part [36, 37]. Metastatic tumors are very common in the late stages of cancer. The spread of metastases may occur via the blood or the lymphatics or through both routes. The most common places for the metastases to occur are the lungs, liver, brain, and bones [38].
The location of the metastases is not always random, with different types of cancer tending to spread to particular organs and tissues at a rate that is higher than expected by statistical chance alone [39]. Breast cancer, for example, tends to metastasize to the bones and lungs.
5.5 Genitourinary System
5.5.1 DTPA Scan
99mTechnetium-labeled diethylenetriaminepentaacetic acid (DTPA) is used to see glomerular filtration rate (GFR). DTPA is a heavy metal chelate cleared through glomerular filtration. Following intravenous injection of 99mTc-DTPA, normal peak cortical uptake occurs by 3–4 min. By 5 min, the collecting system is seen; the bladder is typically visualized by 10–15 min.
5.5.2 DMSA Scan
A DMSA scan is a radionuclide scan that uses dimercaptosuccinic acid in assessing the renal function; it is now the most reliable test for the diagnosis of acute pyelonephritis [40]. The major clinical indications for this investigation are the detection and/or evaluation of a renal scar, the small or absent kidney, an occult duplex system, certain renal masses, systemic hypertension, or suspected vasculitis [41].
5.5.3 Effective Renal Plasma Flow (eRPF)
Effective renal plasma flow (eRPF) is a measure used in renal physiology to calculate renal plasma flow (RPF) and hence estimates renal function:
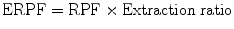 where renal plasma flow (RPF) is the volume of plasma that reaches the kidneys per unit time and extraction ratio is the ratio of compound entering the kidney that got excreted into the final urine.
where renal plasma flow (RPF) is the volume of plasma that reaches the kidneys per unit time and extraction ratio is the ratio of compound entering the kidney that got excreted into the final urine.
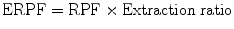
5.5.4 Glomerular Filtration Rate (GFR)
Glomerular filtration rate (GFR) is the volume of fluid filtered from the renal (kidney) glomerular capillaries into the Bowman’s capsule per unit time. GFR can be calculated by measuring any chemical that has a steady level in the blood and is freely filtered but neither reabsorbed nor secreted by the kidneys.
The GFR is typically recorded in units of volume per time, e.g., milliliters per minute ml/min.
There are several different techniques used to calculate or estimate the glomerular filtration rate (GFR or eGFR).
5.5.5 Renovascular Hypertension
Renovascular hypertension or renal hypertension is a syndrome which consists of high blood pressure caused by narrowing of the arteries supplying the kidneys (renal artery stenosis). It is a form of secondary hypertension—a form of hypertension whose cause is identifiable.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




