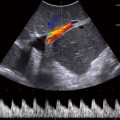
Fig. 21.1
Figure represents needle entry into a collection of unknown etiology using a curved probe for guidance
Informed consent following a thorough discussion with the patient is mandatory for any invasive procedure. Rarely are the procedures emergent and the expectations of the patients and primary-care providers need to be assessed prior to performing the procedure. Identification of the indications, approach to drainage, choosing the appropriate imaging modality, specific diagnostic tests on the drainage, and therapeutic options should all be discussed at this time.
In diagnostic or therapeutic drainage, the steps are fairly simple though difficult to execute without sufficient practice. The collection is identified using an ultrasound probe (as mentioned earlier, the probe used to initially find the collection and that used for targeting drainage may be different). Using ultrasound guidance, the collection is approached using an 18- or 22-gauge entry needle based on the depth and size of the fluid. Advancing the needle in line with the ultrasound transducer provides excellent visualization of the entire needle and the target collection simultaneously. This improves safety and maneuverability in advancing the needle. If purulent fluid is aspirated, it is sent for culture; of note, immunocompromised patients may have bacteria or fungal collections without evidence of an immune response. Keep in mind that a Gram stain may fail to grow any organisms if the patient has already received antimicrobial therapy. Furthermore, use of a needle without the syringe attached can be a useful technique to facilitate guiding the needle into the cavity without the added weight of the syringe. The syringe can be attached once entry into the cavity can be confirmed by imaging. Based on the thickness of the fluid, the needle may need to be “upsized” prior to drainage of the contents.
To “upsize” the needle, a guidewire can be inserted with serial dilation over the guidewire using a Seldinger technique. A 0.010- or 0.018-in. wire can be placed through the 22-gauge needle whereas a 0.035 guidewire can be inserted through an 18-gauge entry needle. When using larger dilators, we routinely exchange a thinner guidewire to a larger diameter and stiffer wire to provide a stiff backbone for the dilator to travel over preventing dislodgment and errant passage of the dilator. Furthermore, the needle and guidewire exchange is easier to accomplish with the cavity filled with fluid as there is more resistance on the wall to prevent dislodgement as well as prevent inadvertent entry through the opposite wall. If the fluid within a cavity has already been evacuated for diagnosis, irrigation with saline can be used to refill the area and help with the needle and guidewire exchange. Once a drainage cathether has been inserted into the cavity, we routinely use ultrasound to confirm adequate drainage of the cavity and assess for additional located collections that were not initially noted or were inadequately drained. Although, the largest possible drainage catheter is generally used, there is no difference in recurrence, drainage time, and complications [3]. Figure 21.2 demonstrates evacuation of a loculated abscess by breaking up septations using the needle and placement of a pigtail for irrigation and drainage.
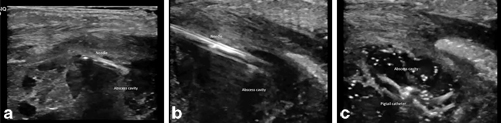

Fig. 21.2
a Figure represents needle entry into a loculated abscess cavity with multiple septations. b Figure represents needle connecting multiple loculated collections by breaking up the septations under visualization. c Figure represents abscess cavity being drained by the pigtail catheter that is now in place. The abscess is irrigated with saline and collapsed down at the end of the procedure
The management of a drain when placed should be standard for each institution. Drains can be placed to bulb or gravity drainage based on the expected quality and quantity of output. They should be ideally flushed with saline daily and removed in about 1–3 days when the output is less than 10 ml/day or 5 ml/day in infants [2]. While a drain is in place, antibiotics should be considered if there is a risk of translocation based on host factors, if there is adjacent foreign material such as an implant, or if the output is presumptively infectious.
Ultrasound has become the imaging modality of choice for many interventions with the advent of high-resolution, wide-angle probes. Ultrasound also offers the unique advantages of portability, immediate availability, and flexibility, owing in part to the wide range of transducers now available. Ultrasound probes have been specifically designed to guide the biopsy of small lesions, allow precise control of biopsy instruments, and provide direct, real-time visualization of successful target lesion biopsies. Moreover, ultrasound avoids ionizing radiation—an important concern especially when imaging the organs of the pelvis, namely the gonads [4].
Transrectal Drainage
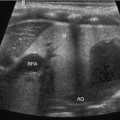
A transrectal approach can be used for drainage of an abscess or fluid collection in the pelvis. As the bowel can obscure the collection and prevent a transabdominal approach to the collection, a transrectal access can be more direct. Also, this approach may be less painful than transgluteal as it does not traverse any muscles. A transgluteal approach requires the catheter to be placed close to the sacrum/coccyx to avoid injury to the sciatic nerve and gluteal vessels; this approach can cause pelvic and leg pain if it passes through the piriformis muscle or near the sacral plexus [4]. Securing the catheter using the transrectal approach may be more difficult though, therefore, a pigtail is recommended. Figure 21.3 demonstrates the use of real-time ultrasound to access a pelvic abscess using the transrectal approach.
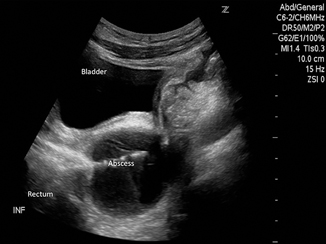

Fig. 21.3
Figure represents needle entry into a pelvic abscess using a transrectal approach. The needle enters the cavity under direct visualization. The probe (curved) was placed in a longitudinal orientation on the abdominal wall visualizing the bladder and rectum while accessing the abscess cavity
One study recommends having all patients who are having transrectal or transvaginal drainage of an abscess be started on antibiotics at least 2 h in advance to achieve adequate systemic levels [5]. A bowel preparation is considered unnecessary for transrectal drainage; however a gentle enema—if not contraindicated—using saline or Fleet’s may help clear out remnant stool, making the procedure easier and cleaner to perform.
For teenagers, a transrectal probe with guide can be used since their anal sphincter complex can accommodate the probe without damage. However, in both older and younger children, a curved transducer in a sagittal orientation placed just above the pubic bone can provide excellent transabdominal guidance for transrectal abscess drainage. In this method the bladder acts as an acoustic window so that catheterizing the bladder to fill with saline augments visualization [5]. Leaving a pigtail catheter in the abscess is preferred to solely draining an abscess when transrectal approach is used. Furthermore, daily irrigation should be performed approximately every 8 h with 5–10 mL of sterile saline to flush both the abscess cavity and the associated tubing [4].
Transrectal techniques are also used in assessment of male infertility, hemospermia, and azospermia. Seminal vesicle, ejaculatory duct, and midline cysts can be aspirated under transrectal ultrasound guidance and analyzed for semen and sperm motility. Aspiration of ejaculatory duct cysts may improve obstructive azospermia and improve paternity rates [4].
Furthermore, the transrectal technique is a safe, adequate, and effective treatment for prostate abscess [6].
The rest of the chapter focuses on diagnostic and therapeutic approaches that should be considered for various anatomic locations.
Head and Neck
Drainage of a fluid collection in the head and neck region can be required for an infection with subsequent abscess formation, hemorrhage into an existing cyst, or a postoperative complication [7]. As the oral cavity is closely intertwined with head and neck anatomy, several sources of bacterial translocation can occur. As these anaerobic organisms spread, they can lead to a retropharyngeal abscess or those that track anteriorly into the mediastinum. Early drainage can identify these organisms and allow for aggressive treatment, preventing later complications [8].
Peritonsillar cellulitis and abscess can present similarly with edema, trismus, and erythema [9]. Usage of ultrasound improves the diagnostic accuracy and ability to drain the collection. As the carotid artery can be near the posterior extent of the abscess, an ultrasound can be used to guide the needle while limiting the potential injury to adjacent structures. In one study, ultrasound was used to diagnose and drain the collection in the emergency room, facilitating an earlier discharge home without complications [10].
Cysts in the head and neck region can be fairly common, arising from the thyroid origin as a thyroglossal duct cyst—a dermoid cyst confined to the superficial tissues or laterally as branchial cleft cysts. These cysts ultimately may require surgical excision; however, infection or hemorrhage can occur at initial presentation or postoperatively requiring ultrasound-guided drainage for management. The most important concepts to consider in the head and neck region include: (1) the use of anatomic landmarks and identification of important vascular and air-filled structures that course over a small area and (2) weighing the risks of drainage for diagnostic purposes versus an earlier more aggressive surgical approach for therapy. Alternatively, use of sclerotherapy after drainage of cysts in the thyroid, parathyroid, and salivary glands has been shown to be successful to prevent recurrence [11]. Figure 21.4 demonstrates entry into a thyroid cyst for diagnostic sampling.
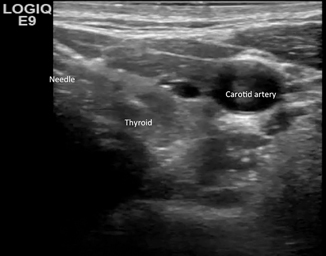

Fig. 21.4
Figure represents needle entry into a cyst in the thyroid. Note the adjacent vascular structures and how well they can be visualized and avoided using ultrasound
Chest
Pleural effusions or fluid collections in the chest can occur for a variety of reasons—infectious, malignant, lymphatic, or idiopathic. Classically, drainage of the fluid has been used for diagnosis—classifying effusions as transudative versus exudative. The Light criteria can be used to define the type of effusion [12]: pH < 7.2, lactate dehydrogenase > 1000 U, glucose < 40 mg/dL, or < 25 % blood glucose, Gram stain, or culture positive and with loculations or septations proven with imaging indicating an exudative or complex collection. In future studies, an exudative effusion was most definitively confirmed with a low pH [13−15]. Figure 21.5 demonstrates the use of ultrasound to drain a pleural fluid collection.
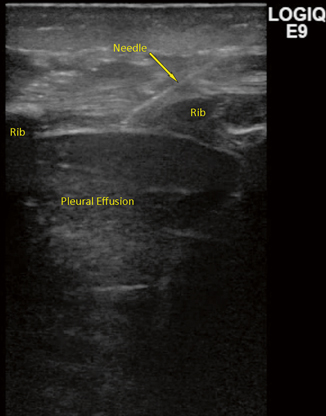

Fig. 21.5
Figure represents needle entry into the pleural cavity using a linear probe. Note the image quality below each rib causing a shadow to appear. The needle usually “disappears” in this shadow and reappears on the other side. The lung is collapsed and therefore seen better than usual when fully filled with air
Parapneumonic effusions occur during or after a bout of bacterial pneumonia, with translocation of the bacteria into the pleural space from the interstitial. The fluid starts as clear with some mild particulate matter, thickening over time, until it reaches a fibrotic nature, compromising the lung volume by trapping the lung in various areas (Fig. 21.6a and b). Drainage of the fluid alone has been used for treatment, but has a high recurrence rate. Surgical approaches with or without using minimally invasive techniques have been advocated to decorticate the fibrotic bands and release trapped lung; however, this carries morbidity of the surgery and an increase in cost. Alternatively, drainage with insertion of a lytic agent has been proposed to decrease the recurrence rate without the morbidity or cost associated with surgery [16]. Placement of the drainage catheter can be performed using an ultrasound to target the area of fluid more precisely.
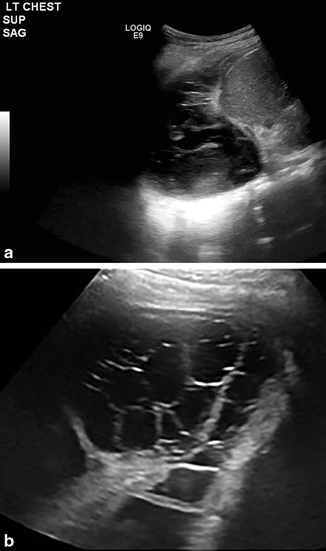

Fig. 21.6
a and b The pictures show possible sonographic findings in the setting of an advanced parapneumonic pleural effusion or empyema . Treatment options include ultrasound-guided placement of pigtail chest tube with TPA(tissue plasminogen activator) applications or VATS (video-assisted thoracoscopic surgery) with decortication
On patients on a ventilator, drainage of simple or complex pleural effusions in mechanically ventilated patients appears to improve oxygenation and is safe [17]. A pleural effusion was detected in critically ill patients in 60 % of patients in one intensive care unit (ICU) with over 40 % present on admission [18]. These effusions can be the result of heart failure, atelectasis, malignancy, pneumonia, hypoalbuminemia, and even positive-pressure ventilation [18, 19]. In any patient where drainage of an effusion is considered, there is a risk of an iatrogenic pneumothorax with intervention. Therefore, the use of an ultrasound should be considered mandatory for the drainage of pleural effusions in this population of patients that has minimal reserve at baseline.
Malignant pleural effusions affect more than 175,000 people each year in the USA, with lung cancer contributing to 30 % of the cases [20]. Thoracentesis or drainage of the pleural fluid can be used for diagnostic and therapeutic purposes when a malignant effusion is suspected. Unfortunately, nearly a third of these procedures may be associated with a complication when performed without an ultrasound, with a third of these complications being a pneumothorax [21




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree