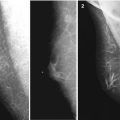
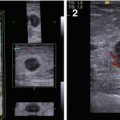
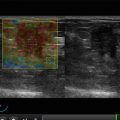
Fig. 7.1
Gynecomastia. (a) (1, 2) Photo. (b) Scheme. (c) (1, 2) Mammography
According to Akimov (1992), one third of men with gynecomastia had lesions diffusely distributed within the breast and presented with small fibrous areas in adipose tissue. Sometimes they contained cysts of 0.5 cm in size with transparent fluid. Approximately 9 % of patients exhibited bilateral changes. Two thirds of cases of gynecomastia were local. The areas were centrally located in the subareolar area, elastic with palpation, had a roundish shape, and ranged in size from 0.7 to 8 cm. All cases of focal gynecomastia were unilateral (Akimov 1992).
Classifications of gynecomastia are based mainly on etiological factors. Thus, Zhukovsky and Lebedev (1982) classify gynecomastia as drug-induced, symptomatic, familial, and false. Eulenburg et al. (1984), considering a principal cause of gynecomastia to be the disturbance in hormonal balance between estrogens and androgens, classify three physiological types of gynecomastia: gynecomastia of newborns, young men, and the elderly. Ostrovskaya et al. (1988) classify gynecomastia as true and false, the first being a breast dishormonal hyperplasia and the second characterized by enlargement of the breast because of excess subcutaneous fat.
Gynecomastia can be a consequence of hormonal imbalance, such as elevated level of estrogen, lowered level of androgen, defect of androgen receptors, and increased sensitivity of mammary tissue to estrogen. Gynecomastia can be physiological or can result from a disease. Of all cases, 25 % are of an idiopathic nature. Classifications of the syndrome of gynecomastia can be based on the causes of imbalance of male and female sex hormones.
One example of classification is:
I.
Physiological gynecomastia
Newborns
Teenagers
In elderly people
II.
True gynecomastia
Idiopathic
Persistent in teenagers
Familial
III.
Symptomatic gynecomastia
Hormone-secreting tumors (e.g., tumors of testicles or various organs (lung, liver, intestine) that secrete chorionic gonadotropin)
Endocrine diseases, including genetic (e.g., true hermaphroditism, Klinefelter syndrome, congenital adrenal hyperplasia, Kennedy’s disease, Graves’ disease)
Primary hypoandrogenism (e.g., infectious, granulomatous orchitis, anorchism, castration)
Renal and/or hepatic failure
Medicinal (estrogen, human chorionic gonadotropin, spironolactone, flutamide, cardiac glycosides, tricyclic antidepressants, opiates, etc.)
IV.
False gynecomastia
Adipose breast tissue hyperplasia
V.
Breast tumors
Malignant (cancer or sarcoma)
Benign (adenoma, lipoma, fibroma, etc.)
Physiological gynecomastia exhibits three age peaks: in newborns, during puberty, and in the elderly.
A classification based on the stability of pathological hyperplasia (Serra-Diaz et al. 1999) is:
1.
Neonatal.
2.
Pubertal (transitional) gynecomastia as a physiological phenomenon is observed in 4–9 % of boys in puberty and disappears after puberty.
3.
Persistent pubertal gynecomastia is considered abnormal and does not exhibit spontaneous regression.
Sotnikov and Baytinger (2006) suggest four degrees of severity of gynecomastia:
I.
Minimum subareolar nodosity
II.
Subareolar mass smaller than the diameter of the areola
III.
Mass equal to the diameter of the areola
IV.
Mass larger than the diameter of the areola
In surgical practice, gynecomastia division into nodular, diffuse, and mixed is considered most convenient.
Gynecomastia is also classified in accordance with its dimensions into the following groups: moderate, medium, and expressed. The formula, C × H ÷ 2, where C is the circumference of the breast (in centimeters) and H is the height of the breast (in centimeters), is used to define the groups; moderate gynecomastia corresponds to <6 cm2 (incidence ~10 %), medium is 6–10 cm2 (incidence ~71 %), and expressed is >10 cm2 (incidence ~18 %).
Gynecomastia of newborns is a secondary event related to transplacental transmission of hormones into the fetus. It is observed in 60–90 % of all newborns. It is characterized with infiltration in the subareolar region, sometimes accompanied with colostrum. It spontaneously passes within several days or weeks and does not require any medical influence.
Gynecomastia in puberty is reported in 4–6 % of adolescents. It is usually bilateral and asymptomatic. In most cases, it spontaneously passes within several months or years. In some cases, it can be significant and last for an extended period because of the sensitivity of the mammary tissue to estrogen stimulation.
During early puberty, the level of estrogens increases faster than testosterone, inducing an imbalance of the estrogen/androgen ratio. During the formation of the sexual system, the secretion of follicle-stimulating hormone (FSH) and the aromatase activity of testicular Sertoli cells increase against a low level of luteinizing hormone (LH), which should stimulate the hormonopoiesis of testosterone in Leydig cells. The latter reaches the level of adult men only some years later. Pubertal gynecomastia with histology shows the changes in the interstitial tissue with insignificant proliferation of ductal epithelium. It requires conservative or surgical treatment in severe cases. To exclude other than physiological causes of gynecomastia, the patient should consult a urologist and undergo examinations of the liver and intestines (the organs and systems participating in hormone metabolism). Pubertal gynecomastia usually spontaneously disappears in 6–18 months. A small enlargement of the breast does not require any treatment in the majority of teenagers.
Persistent gynecomastia is a pathological condition. After puberty, the remaining enlargement of the breast requires surgical treatment, if conservative treatment fails to improve the situation. Endocrinologic examination permits the classification of persistent gynecomastia into four variants: normopubertal, hypopubertal, hyperpubertal, and femininopubertal.
Gynecomastia of elderly people can be observed in men older than 60 years. The abnormality results from fading of testicular function leading to a change in the hormone ratio (a relative elevation of estrogen levels). The diagnosis is applicable if other causes of gynecomastia are excluded.
Iatrogenic gynecomastia in adult men is reported most often. More than 120 drug groups can induce gynecomastia, such as estrogen, steroids, glucocorticoids, and gonadotropins. This type of gynecomastia is reversible and regresses after drug cessation. Gynecomastia in endocrine and nonendocrine diseases can accompany liver or kidney pathology. It is a consequence of disturbance in metabolism and excretion of steroid hormones and prolactin.
Gynecomastia may periodically accompany diabetes (Sencha et al. 2013a, b). The main causes are considered disturbances in liver and kidney functions. Diffuse diabetic glomerulosclerosis usually appears 4–5 years after diabetes manifestation, and 15–20 years from the onset of the disease, these changes are observed practically in all patients with diabetes. The nodular affection of the renal glomulus is often observed. It starts almost from the very beginning of diabetes and quickly progresses, resulting in glomerulocapillary microaneurysms, which narrow or completely obstruct the capillaries. It leads to the accumulation of toxic products and disturbance in the destruction of peptide hormones. It subsequently results in their accumulation in the blood, with influence on sexual glands and directly on breast tissue, with the growth of the ductal tree and development of gynecomastia. There is an opinion that gynecomastia can be a sign of functional abnormalities of the pituitary–sexual gland system in diabetic men because of progressing insufficiency of the microcirculation.
Gynecomastia in men with prolactinoma may accompany galactorrhea and hyperprolactinemic hypogonadism. Treatment of a tumor of the pituitary gland, conservative (dopamine agonists) or adenomectomy, leads to involution of gynecomastia. Gynecomastia in subthalamic dysfunction is caused by a disturbance in the regulation of adenohypophysis with the change in production of prolactin, LH, or/and FSH. Gynecomastia can accompany hyperthyroidism in children and be the first sign of hyperthyroidism in adult men because thyroid hormones activate steroidogenesis. Hypothyroidism may also be accompanied by gynecomastia, when hyperstimulation of the pituitary gland arises under the influence of excess thyroliberin. This is characteristic for primary hypothyroidism. Correction of hypothyroidism leads to the regression of gynecomastia. In men with cryptorchism, anorchism, and ineffective spermatogenesis and synthesis of testosterone and after testicular trauma or autoimmune processes in testicles with their atrophy, hormone replacement therapy is a method of treatment and gynecomastia preventive maintenance. Gynecomastia appears in genetic diseases with endocrinopathies (hyperprolactinemia and hypersensitivity of breast tissues to estrogens in Klinefelter syndrome and increase in the production of estrogens in Reifenstein syndrome and testicular feminization syndrome).
Gynecomastia in nonendocrine diseases often accompanies severe pathology of the liver and kidneys, such as hepatitis, liver cirrhosis, and chronic renal insufficiency. The method of choice for treatment in such cases is surgical because drug administration can negatively affect the pathological processes.
Kidney diseases with so-called uremic hypogonadism are also associated with gynecomastia. Chronic renal insufficiency with suppression of glomerular filtration and increase in blood urea, creatinine, and other nitric slags leads to a decrease in the secretory activity of Leydig cells. At the same time, gynecomastia can be related to chronic renal insufficiency because the kidneys are the location of the degradation of peptide hormones (prolactin, gonadotropin). A disturbance in the process of their destruction results in their accumulation in blood and their influence on sex glands and breast tissue. Breast enlargement may be observed in men with chronic renal insufficiency on hemodialysis. The duration of hemodialysis does not always matter for the development of gynecomastia. However, gynecomastia often arises within the first weeks of hemodialysis and spontaneously disappears in most cases.
The understanding of the connection of gynecomastia with prostate diseases is enhanced by data regarding the increase in the conversion of testosterone to dihydrotestosterone by hypertrophied prostates. The close interrelation between the prostate and sexual glands in the development of gynecomastia is based on the interaction of secretory functions of these organs. Thus, a decrease in the function of the prostate is accompanied by testicular atrophy and abnormal spermatogenesis. An enhanced secretory activity of the prostate suppresses the activity of testicles, whereas the reduction of its secretion stimulates their function.
Gynecomastia as a paraneoplastic syndrome can arise in cases of hormone-secreting tumors. It is often the first and the single sign of a tumor (lung, pancreatic, liver, kidney, testicular cancer) for a long time. Breast enlargement is induced by ectopic secretion of estrogens or gonadotropins by the tumor and, hence, typically bilateral. Gynecomastia may be observed in cases of benign hormone-active tumors in any age (feminizing adrenal or testicular tumors).
Gynecomastia is idiopathic in 25 % of cases, and the cause is left undetermined. Gynecomastia can be unilateral or bilateral and symmetric or asymmetric. Obesity in men leads to breast enlargement caused by adipose tissue hyperplasia (pseudo-gynecomastia). True gynecomastia is defined with ultrasound (US) by the presence of subareolar infiltration, which can be of different density and structure. There are several types of gynecomastia, such as nodular (in the form of roundish intense homogeneous lesion), treelike (in the form of wide dense fibrous branches), and diffuse glandular (an appearance similar to mastopathy in women). Diffuse gynecomastia presents clinically with a painful swelling of one or both breasts, in some cases with nipple discharge, coarse lobularity, and multiple foci that have a changeable character. Nodular gynecomastia more often affects one breast and is constant with a tendency toward enlargement.
The risk of breast carcinoma depends on the expression of ductal, lobular, or intracystic proliferation. Based on the degree of proliferative activity of epithelium, the following types are specified (Letyagin 2006):
Gynecomastia without proliferation (the risk of development of breast cancer is 1.5 times higher)
Gynecomastia with epithelial proliferation (the risk of malignancy increases up to 1.9 times)
Gynecomastia with atypical epithelial proliferation (3 times increase in risk, up to 25 times according to some publications)
US often detects the following features in all types of gynecomastia (Fig. 7.2):
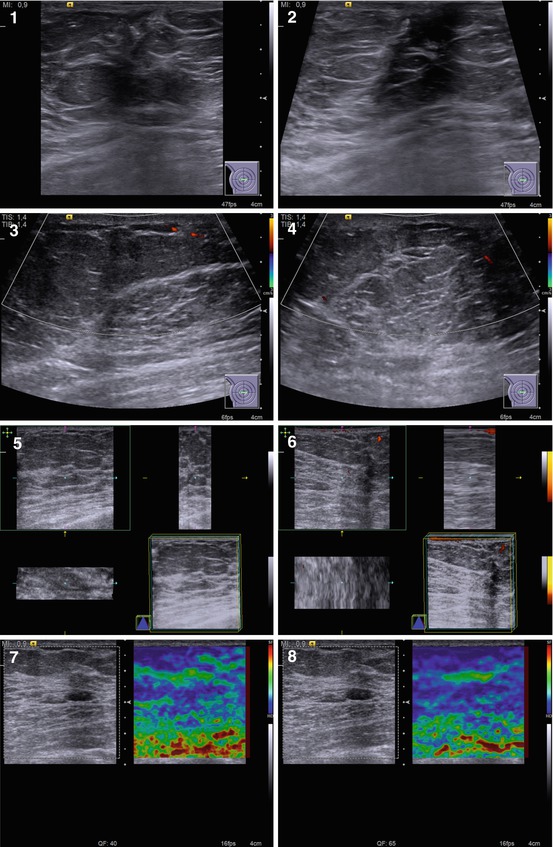

Fig. 7.2
(1–8) Gynecomastia. Grayscale US, CDI, 3D, and US elastography
Hypoechoic area or lesion
Central retroareolar location within the breast
Irregular shape
Accurate or indistinct contours, rough borders
Various dimensions from 5–7 mm to 25–30 mm
Hypovascular or avascular with color Doppler imaging (CDI), power Doppler imaging (PDI), and three-dimensional power Doppler vascular image reconstruction (3DPD)
Heterogeneous medium-grained color pattern with US elastography similar to the surrounding tissues
Average shear-wave velocity of 2.4 m/s with acoustic radiation force impulse (ARFI) (Virtual Touch™ tissue quantification (VTQ)) and average strain ratio in different aspects not differing from those in healthy breast tissues
Fourteen percent of men with gynecomastia in our study exhibited the nodular type with the following features (Fig. 7.3):
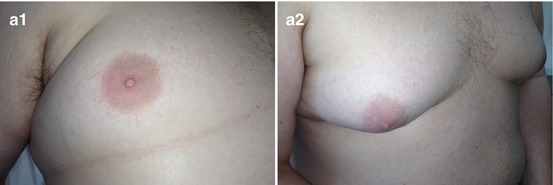
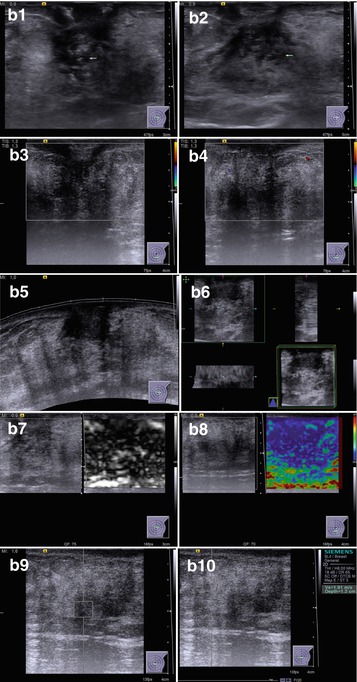
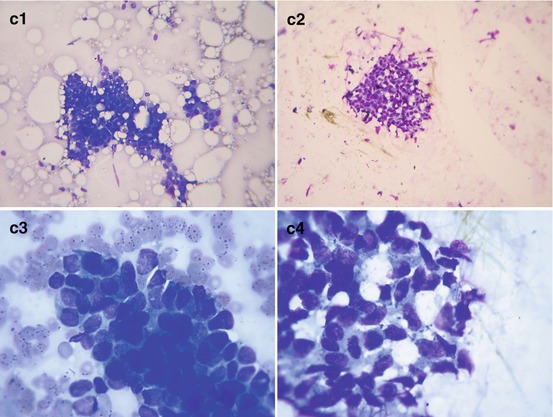



Fig. 7.3
Nodular gynecomastia. (a) (1, 2) Breast view. (b) (1–10) Grayscale US, CDI, panoramic scan, 3D, US elastography, and ARFI. (c) (1–4) Hematoxylin–eosin-stained smears. Original magnifications ×100 and ×400
A lesion of 1.2–3.8 cm in size against fibroadipose tissue in retropapillary area
Irregular shape (74.6 %)
Decreased echodensity
Heterogeneous structure (anechoic component was observed in 59.7 % of cases)
Indistinct, rough contours
Hypovascularity with CDI, PDI, and 3DPD (68.4 %)
Heterogeneous large/medium-grained color pattern with US elastography almost identical to the surrounding structures
Average shear-wave velocity of 2.4 m/s with ARFI (VTQ) and average strain ratio in different aspects not differing from those in healthy breast tissues
The treelike type of gynecomastia was observed in 32.3 % of patients. US defined wide dense fibrous linear structures in some breast areas (most often retroareolar) with a heterogeneous echostructure (an anechoic component was observed in 11.5 % of cases), and avascular with CDI, PDI, and 3DPD (Fig. 7.4). The diffuse type was reported in 53.7 % of men with gynecomastia. It was characterized by diffuse changes of various degree of manifestation throughout the whole breast without solid or cystic lesions (Fig. 7.5).
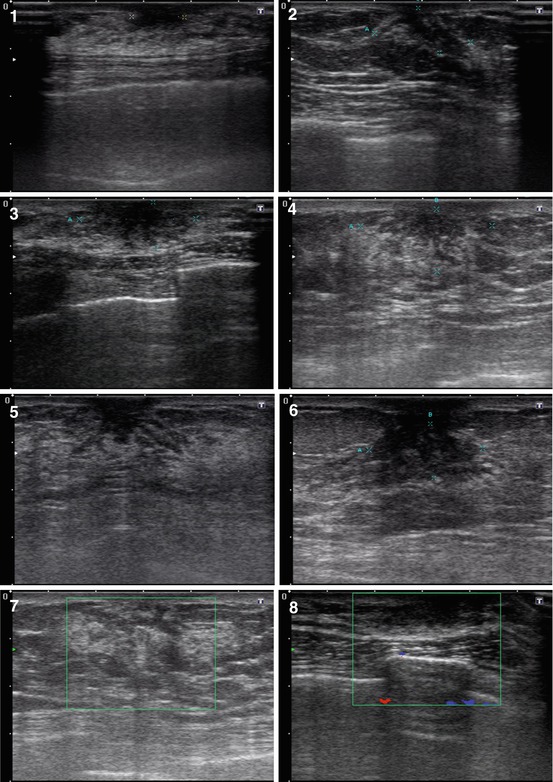
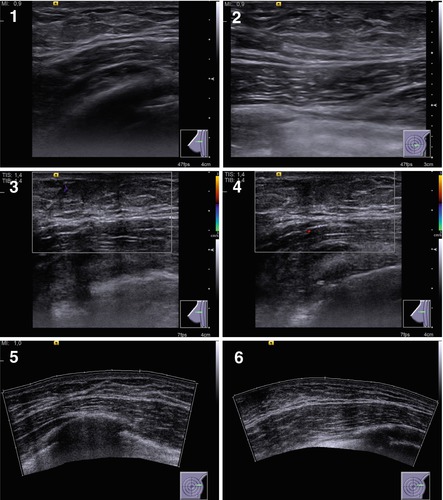
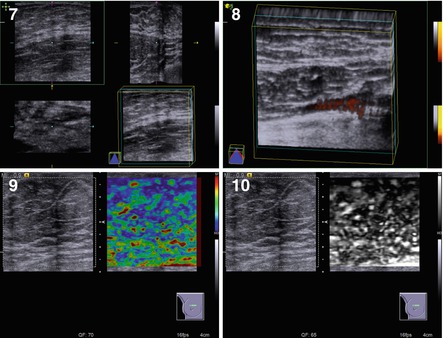

Fig. 7.4
(1–8) Treelike gynecomastia. Grayscale US, CDI, panoramic scan, 3D, and US elastography


Fig. 7.5




(1–10) Diffuse glandular gynecomastia. Grayscale US, CDI, panoramic scan, 3D, and US elastography
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree