Diffusion tensor imaging (DTI) and perfusion-weighted imaging (PWI) are essential tools for diagnosing, differentiating, and monitoring brain tumors. High-field MRI provides higher signal-to-noise ratio, shorter scan time, and better image quality. One-stop multiparametric study, including DTI and PWI, is possible with high-field MRI in brain tumors. DTI can be used for assessing spatial relationship between major white matter tract and tumor, differentiating gliomas from nonglial tumors, and postoperative evaluation. PWI provides reliable biomarkers for glioma grading, therapeutic responses, and differential diagnosis of various brain tumors. With higher field strength, better-quality DTI and PWI can raise the diagnostic accuracy in brain tumors.
- •
High-field MRI provides higher signal-to-noise ratio, shorter scan time and higher resolution images.
- •
Multiparametric imaging is possible with high-field MRI, which includes diffusion tensor imaging (DTI), perfusion-weighted imaging (PWI), functional MRI, susceptibility-weighted imaging, and MR spectroscopy.
- •
DTI and PWI are essential diagnostic tools for grading glioma, differentiating glioma from nonglial tumor, and therapeutic monitoring of brain tumors.
- •
One-stop multiparametric MR protocol is recommended for initial assessment of brain tumors.
- •
Clinical usefulness of diffusion tensor imaging (DTI) and perfusion-weighted imaging (PWI) was proven and initial MR protocol should include them.
- •
DTI and PWI are reliable biomarkers of glioma grading, differentiation from nonglial tumors, and monitoring of treated glioma.
- •
Optional fluid attenuated inversion recovery, permeability imaging, functional MRI, and MR spectroscopy are recommended in specific cases.
In brain tumor imaging, the multimodality approach is favored for the differential diagnosis of brain tumors and grading of gliomas. Simultaneous acquisition of routine imaging with perfusion, diffusion, functional MRI (fMRI), and MR spectroscopy enables a better diagnostic approach to brain tumors. High-field MRI enables shorter scan time with satisfactory image quality compared with the low-field system, and therefore the high-field system is more suitable for multimodality imaging.
High-field MRI has some disadvantages, such as increased susceptibility artifacts, poorer gray-white matter differentiation on T1-weighted imaging, and increased specific absorption rates. Better blood oxygen level dependent effect in fMRI and susceptibility-weighted imaging is a good example of counterplot to increase susceptibility effect in high-field MRI. Various solutions have been proposed for apparent T1 contrast of brain tissue and decreasing specific absorption rates during MR examination. With these advantages, high-field MRI is not paralleled by any imaging modality in brain tumor diagnosis. This article describes two advanced imaging techniques performed on clinical 3 T MRI: diffusion tensor imaging (DTI) and perfusion-weighted imaging (PWI).
DTI of brain tumors in high-field MRI
As for DTI, its increased resolution provides a lesser partial volume averaging effect and higher SNR, resulting in more accurate fiber tracking. Increased matrix size and isovoxel acquisition is much more useful for clinical fiber tracking, especially for identifying complex fibers such as optic radiation ( Fig. 1 ), and for detailed anatomic structures such as brain stem nuclei and gray matter. Even though echo planar imaging is extremely susceptible to magnetic field inhomogeneity and image distortion is more apparent with high-field MRI, several solutions have been suggested and implemented to commercial scanners.
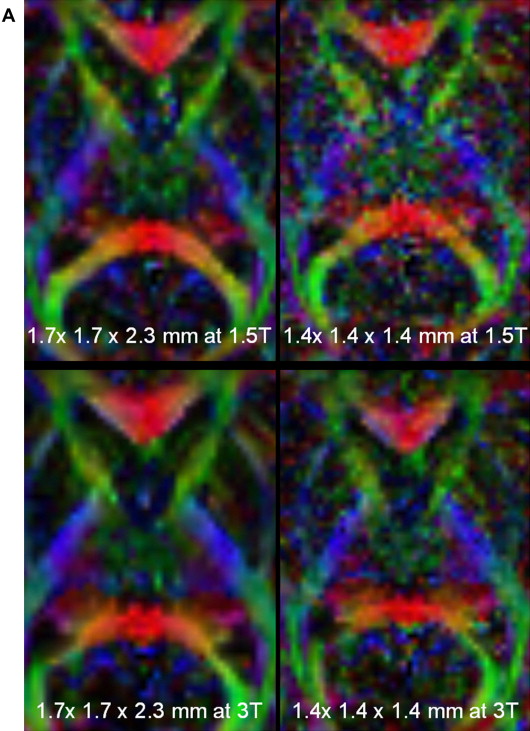
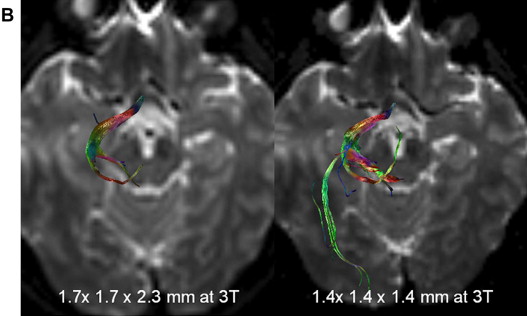
DTI provides a quantitative biomarker of brain tissue, fractional anisotropy (FA), and apparent diffusion coefficient (ADC), which are used in the differential diagnosis of brain lesions. White matter fiber tracking is possible based on FA and trajectory of voxels. Two different methods are applied in the calculation of white matter tracts. The first one is a deterministic approach called fiber assignment by continuous tracking (FACT). In clinical practice, the FACT method is preferred to probability maps because it is more intuitional and gives direct information to surgeons. However, it has some limitations, such as simple calculation from a seed point, inability to solve fiber-crossing problems, and multiplicity of tracts’ connectivity. The probabilistic approach is more scientific, considering multiple fiber connectivity from one region to the others. With any approach, DTI and fiber tracking are essentials tool in preoperative localization of brain tumors and white matter tracts and in determining surgical route for adequate resection.
DTI for Differential Diagnosis and Grading of Brain Tumors
Nontumorous lesions, such as tumefactive multiple sclerosis or demyelinating diseases, should be differentiated from tumors. With the aid of gadolinium enhancement (open-ring or incomplete-ring sign) and CT, tumor can be differentiated easily in most cases. However, nonenhancing glioma, such as brain stem glioma, in young people is difficult to be differentiated from demyelinating lesions. On DTI, architecture of corticospinal tract is likely to be preserved while demyelinating diseases show disruption of the arrangement of the tracts on fiber tracking.
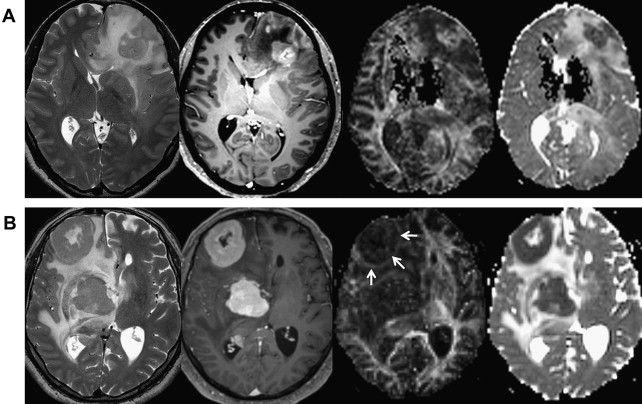
ADC and FA can be used in differential diagnosis of brain lesions. Integrity of peritumoral white matter is altered in high-grade gliomas, but not in low-grade gliomas or metastasis. FA is significantly lower and ADC is higher in peritumoral edema in cases of brain metastasis. All of these results are related to microscopic infiltration of tumor cells into edematous regions in glioma, whereas only vasogenic edema occurs in metastasis. FA is significantly increased and ADC is decreased in enhancing areas of glioblastoma compared with low-grade gliomas, lymphomas, or metastasis ( Fig. 2 ). In contrast, another recent study of glioblastoma on 3 T MRI proved that FA of 0.2 or less is well correlated with lower progression-free survival rate. Although FA and other DTI metrics are objective and easily calculated in most cases of brain tumors, their clinical meaning requires more investigation.
DTI for Preoperative and Postoperative Evaluations
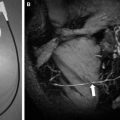
The most powerful aspect of DTI and fiber tracking is preoperative evaluation in space-occupying lesions of brain. Spatial relationships between major white matter tract and brain tumor can be easily identified on DTI. In brain tumors, white matter tract can be invaded or displaced by the lesion. Corticospinal tract and arcuate fasciculus are the most frequently studied structures because they are critically related to motor and language function ( Figs. 3 and 4 ).
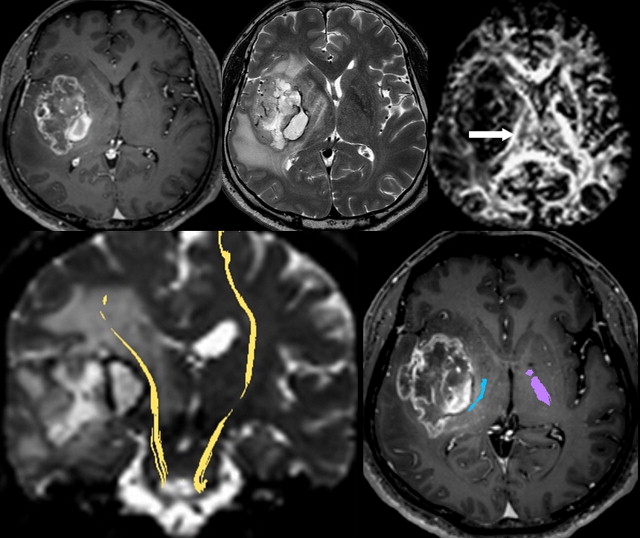
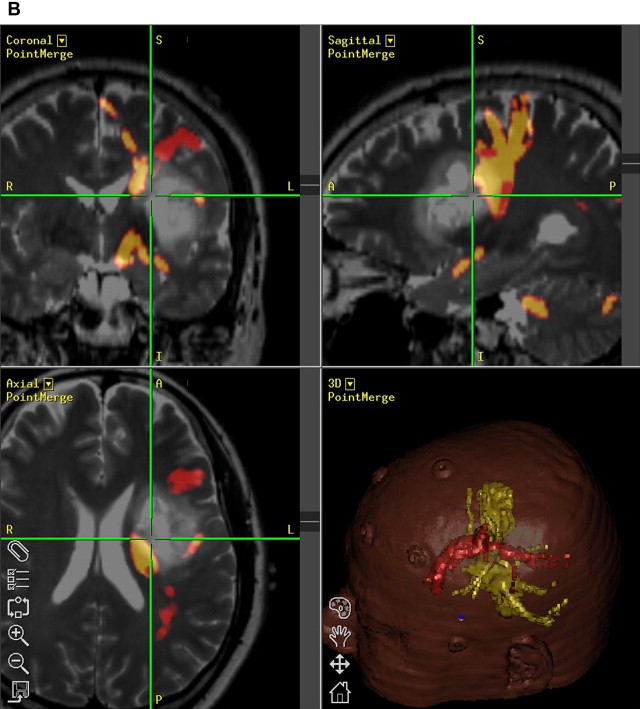
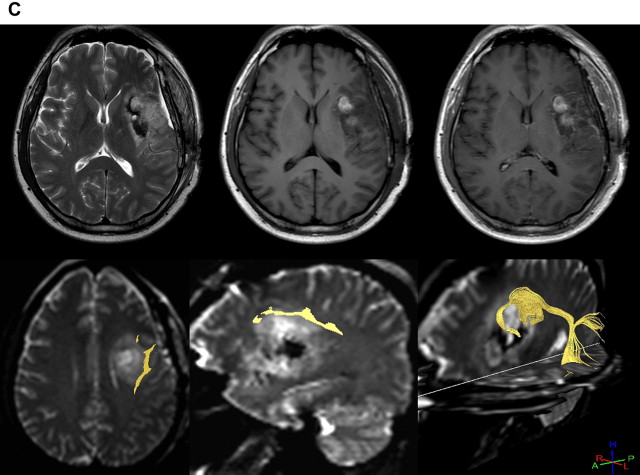
DTI data can now be transferred to a neuronavigation system and used intraoperatively. Geometry of major tract can be shifted from its original position after craniotomy or resection of brain tissue, and this should be taken into account to avoid inevitable injury. In cases of postoperative complication, DTI can be used to assess for injury to white matter tracts and to predict prognosis.
Alteration of brain connectivity after surgery or chemotherapy is related to postoperative changes in cognitive function. In pediatric posterior fossa tumors, the cerebello-cerebral connection can be altered after surgery. Impairment of cognitive function after cerebellar surgery can be explained through DTI findings describing altered cerebello-cerebral connectivity.
PWI of brain tumors in high-field MRI
High-field MRI enables multiparametric MR evaluation of brain tumors within a clinically feasible scan time and better image quality. Conventional imaging with gadolinium enhancement is enough in some tumor cases, but advanced studies are mandatory for precise evaluation of tumor infiltration to adjacent white matter, grading of glioma, and differentiation of gliomas from nonglial tumors, because the therapeutic plan is different in those cases.
PWI can be performed three different ways in clinical practice: dynamic susceptibility contrast (DSC) enhanced PWI, arterial spin labeling (ASL) PWI, and dynamic contrast-enhanced (DCE) PWI. With long history and accumulated experiences, DSC PWI is widely accepted as the preferred diagnostic tool in grading of gliomas, differentiation of radiation necrosis, and discrimination of gliomas from metastasis or lymphoma. In contrast with ASL or DCE PWI, calculation of relative changes in cerebral blood volume (rCBV) is simple, and most MRI vendors provide user-friendly image processing tools, although absolute quantification of cerebral blood flow needs a more robust process.
DSC PWI
After injection of bolus gadolinium contrast, perfusion parametrics are obtained based on time-concentration curve and indicator dilution theory. Integration of time-relaxation curve after gamma fitting results in rCBV. Cerebral blood flow is obtained after calculation of arterial input function and mean transit time.
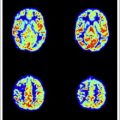
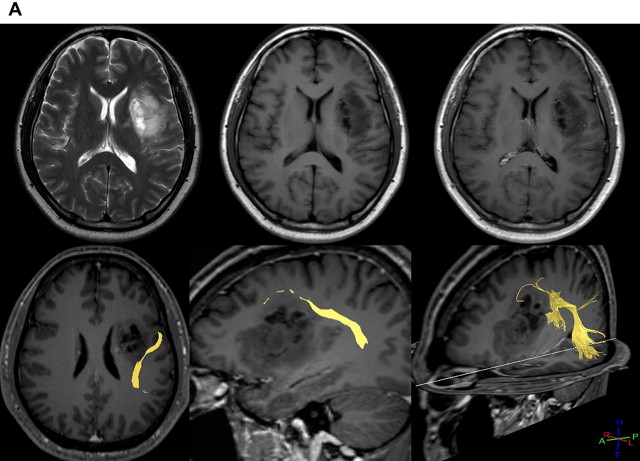
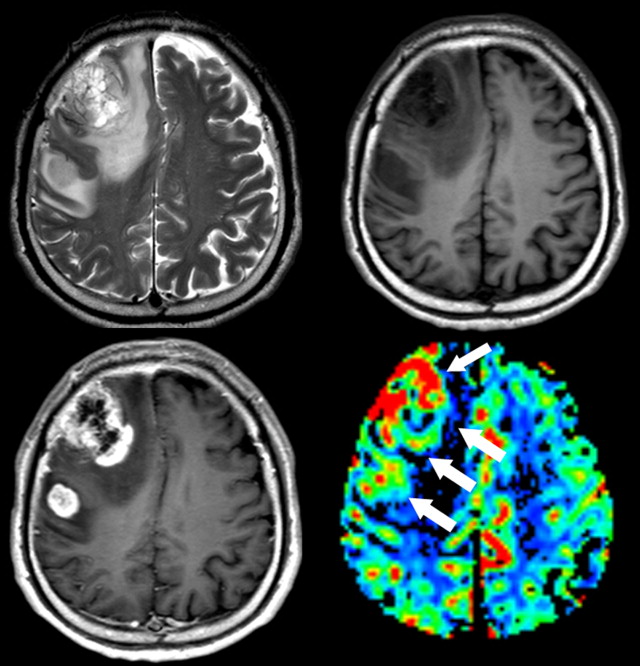
In general, high-grade lesions have larger cerebral blood volume and higher cerebral blood flow. High-grade gliomas have larger rCBV than low-grade gliomas, and lymphomas are likely to have smaller rCBV than gliomas or metastasis. In peritumoral white matter, rCBV is increased in primary glioma because of microscopic cell infiltration, whereas metastasis shows smaller rCBV from pure vasogenic edema ( Fig. 5 ). This finding is similar to FA changes at the peritumoral region on DTI analysis.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree