Etiology
Biliary dilatation can occur as a result of biliary obstruction, from an altered functional state (e.g., after cholecystectomy or with sphincter of Oddi dysfunction), or uncommonly as a result of a choledochal cyst. The role of imaging is to identify a bile duct obstruction and define its level and cause. The cause may be intraluminal, mural, or extrinsic ( Figure 52-1 ). Cholangiographic and cross-sectional modalities provide complementary information, and both are required to investigate biliary dilatation.
The pattern of biliary dilatation depends on the cause and level of abnormality within the biliary tree. The biliary tract may be involved diffusely or focally, or dilatation may be multifocal. Adenocarcinoma of the pancreatic head or a stone in the distal common bile duct (CBD) results in generalized intrahepatic and extrahepatic bile duct dilatation to the level of obstruction. Peripheral cholangiocarcinoma may cause segmental dilatation of the intrahepatic bile ducts. Sclerosing cholangitis and Caroli’s disease typically cause multifocal dilatation of intrahepatic bile ducts.
There are numerous causes of benign and malignant bile duct dilatation. Benign causes include the following:
- •
Choledocholithiasis: Stones in the bile ducts or cystic duct (Mirizzi’s syndrome)
- •
Benign stricture resulting from:
- •
Previous surgery (e.g., cholecystectomy), trauma, instrumentation, or irradiation
- •
Inflammation: Primary sclerosing cholangitis (PSC), pancreatitis, previous passage of a stone, or previous perforated duodenal ulcer; and infection
- •
Miscellaneous: Crohn’s disease, cystic fibrosis with liver involvement, and eosinophilic cholecystitis
- •
- •
Extrinsic compression: Liver cyst, aneurysm ( Figure 52-2 )
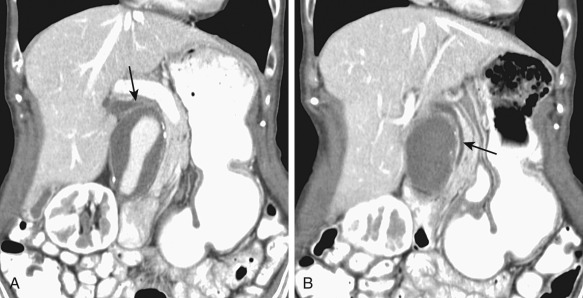
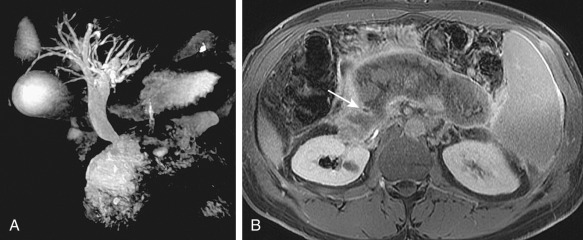
Figure 52-2
Mild biliary dilatation secondary to extrinsic compression of the common bile duct by an abdominal aortic aneurysm. A 66-year-old woman presented with weight loss and elevated alkaline phosphatase level. A and B, Coronal reformatted computed tomography images show mild compression of the bile duct (arrows) by a 4.8-cm infrarenal abdominal aortic aneurysm.
- •
Benign neoplasms: Biliary cystadenoma ( Figure 52-3 ), ampullary adenoma, intraductal papillary mucinous adenoma of the bile ducts

Figure 52-3
Biliary cystadenoma causing mild intrahepatic bile duct dilatation as a result of extrinsic compression. Coronal single-shot fast spin echo (A), postcontrast axial flexible appearance modeling environment (B), and axial T2-weighted fast spin echo (C) magnetic resonance images show a large cystic lesion with enhancing papillary projections ( arrows, A and B ), causing mild intrahepatic bile duct dilatation ( arrow, C ).
- •
Congenital anomaly: Choledochal cyst ( Figure 52-4 ), Caroli’s disease

Figure 52-4
A to D, Postcontrast axial computed tomography sections of the abdomen show a type 1 choledochal cyst (arrow). A stone is noted within the gallbladder.
Malignant causes of bile duct dilatation include the following:
- •
Pancreatic head carcinoma
- •
Cholangiocarcinoma
- •
Ampullary carcinoma
- •
Gallbladder carcinoma
- •
Hepatocellular carcinoma
- •
Malignant mucinous neoplasm of the pancreas or bile duct
- •
Lymphoma ( Figure 52-5 )

Figure 52-5
Post–liver transplant lymphoproliferative disorder. Axial T2 (A), postcontrast T1 (B), and magnetic resonance cholangiopancreatography maximum intensity projection (C) images show a large irregular mass causing left lobe intrahepatic bile duct dilatation. Percutaneous transhepatic cholangiogram (D) via puncture of a dilated left-sided duct shows an abrupt transition point because of the tumor (arrow). E, An 8-French internal-external biliary catheter has been positioned through the tight stenosis.
- •
Metastatic disease
In this chapter, we discuss an approach to biliary dilatation, emphasizing the distinguishing features of benign and malignant causes. We describe some common benign causes of bile duct dilatation. Malignant and benign neoplasms that can cause bile duct dilatation and choledochal cysts are discussed separately.
Prevalence and Epidemiology
Bile duct dilatation can be arbitrarily defined as a common duct diameter greater than 6 mm and an intrahepatic duct diameter greater than 3 mm ( Figure 52-6 ). There is some increase in size of the normal extrahepatic duct with increasing age.
The most common cause of benign biliary dilatation is choledocholithiasis. In Western society, cholesterol stones are the most common type; in Southeast Asia, pigment stones are more common. Parasitic infection of the bile ducts is endemic in Southeast Asia. This precipitates hepatolithiasis and can be complicated by recurrent pyogenic cholangitis.
The exact prevalence of iatrogenic biliary stricture is unclear. Bile duct injury complicates 3 to 7 per 1000 laparoscopic cholecystectomies. Biliary stricture is the most common late complication of hepatobiliary surgery.
The most common malignant cause of bile duct dilatation is pancreatic adenocarcinoma, followed by cholangiocarcinoma. Seventy percent of pancreatic adenocarcinomas involve the head of the pancreas, where they may cause CBD dilatation in addition to pancreatic duct dilatation. The incidence of cholangiocarcinoma is at least four times less than pancreatic carcinoma. Cholangiocarcinoma complicates several benign diseases of the bile ducts, including choledochal cysts, PSC, and parasitic liver infestation. Thus, malignant and benign causes of biliary dilatation may coexist and differentiating one from the other may be problematic.
Clinical Presentation
All causes of bile duct dilatation lead to cholestatic symptoms once obstruction becomes severe enough. The classic symptoms and signs are jaundice, pruritus, pale stools, and dark urine. Typically, choledocholithiasis is associated with acute intermittent right upper quadrant pain and transient jaundice. Malignant causes of bile duct obstruction, such as carcinoma of the head of the pancreas, are often initially painless or manifest as dull pain, and jaundice is progressive. Occasionally, patients with bile duct strictures may develop manifestations of chronic cholestasis: xanthomas, anorexia, nausea, vomiting, weight loss, and deficiencies of calcium and fat-soluble vitamins.
Patients with biliary obstruction typically have elevated serum alkaline phosphatase and gamma-glutamyl transpeptidase levels. These enzymes are disproportionately elevated compared with serum transaminases. In more severe cases of biliary obstruction, total and conjugated bilirubin values are increased.
Patients with elevated levels of alkaline phosphatase and direct hyperbilirubinemia without biliary dilatation have intrahepatic cholestasis with blockage of biliary capillaries as a result of hepatocyte swelling.
Complete or partial bile duct obstruction leads to bile stasis. This can be complicated by ascending cholangitis and stone formation. Ascending cholangitis can be recurrent and life-threatening: the classic clinical manifestation is fever and rigors, jaundice, and right upper quadrant abdominal pain (Charcot’s triad). Patients also may have altered mental status and hypotension (Reynolds’ pentad). Benign causes of bile duct obstruction are more likely to cause ascending cholangitis than malignant causes.
Clinical Presentation
All causes of bile duct dilatation lead to cholestatic symptoms once obstruction becomes severe enough. The classic symptoms and signs are jaundice, pruritus, pale stools, and dark urine. Typically, choledocholithiasis is associated with acute intermittent right upper quadrant pain and transient jaundice. Malignant causes of bile duct obstruction, such as carcinoma of the head of the pancreas, are often initially painless or manifest as dull pain, and jaundice is progressive. Occasionally, patients with bile duct strictures may develop manifestations of chronic cholestasis: xanthomas, anorexia, nausea, vomiting, weight loss, and deficiencies of calcium and fat-soluble vitamins.
Patients with biliary obstruction typically have elevated serum alkaline phosphatase and gamma-glutamyl transpeptidase levels. These enzymes are disproportionately elevated compared with serum transaminases. In more severe cases of biliary obstruction, total and conjugated bilirubin values are increased.
Patients with elevated levels of alkaline phosphatase and direct hyperbilirubinemia without biliary dilatation have intrahepatic cholestasis with blockage of biliary capillaries as a result of hepatocyte swelling.
Complete or partial bile duct obstruction leads to bile stasis. This can be complicated by ascending cholangitis and stone formation. Ascending cholangitis can be recurrent and life-threatening: the classic clinical manifestation is fever and rigors, jaundice, and right upper quadrant abdominal pain (Charcot’s triad). Patients also may have altered mental status and hypotension (Reynolds’ pentad). Benign causes of bile duct obstruction are more likely to cause ascending cholangitis than malignant causes.
Pathophysiology
Certain variants in bile duct anatomy, particularly around the gallbladder fossa, may lead to inadvertent bile duct injury and subsequent bile duct dilatation. The right posterior duct is frequently aberrant. It may drain via the cystic duct, and if this is not recognized at the time of cholecystectomy, bile leak and obstruction can occur.
The anatomy of the junction of the distal CBD and main pancreatic duct is slightly variable. In 60% of cases there is a true common channel at the ampulla of Vater. In 38% of cases there is a “double-barreled” opening at the apex of the papilla. In 2% there are separate duodenal openings for the two canals. Diseases that cause both bile and pancreatic duct dilatation either involve the common channel (e.g., ampullary carcinoma) or are large enough to involve both ducts (e.g., pancreatic carcinoma). Lesions that arise from the wall of bile duct (e.g., cholangiocarcinoma) or are eccentric to the common channel (e.g., duodenal carcinoma) are more likely to manifest as bile duct dilatation without pancreatic duct dilatation. Pancreas divisum or a residual duct of Santorini to the minor papilla may spare the pancreatic duct from dilatation by some periampullary lesions.
Pathology
The pathogenic basis of bile duct dilatation varies. Choledocholithiasis usually is due to bile stasis and supersaturation of one of the constituents of bile. Benign bile duct strictures are often precipitated by an injurious agent that incites an inflammatory response, which is followed by fibrosis. Ischemia plays a role in the development of some strictures (e.g., after liver transplantation [ Figure 52-7 ]), and some strictures are idiopathic (e.g., PSC).
Choledochal cysts are congenital anomalies secondary to an intrinsic weakness in the wall of the bile ducts. Caroli’s disease is an autosomal recessive inherited condition in which there is abnormal embryologic remodeling of bile ducts.
Imaging
The role of imaging is to establish the presence of biliary dilatation and determine the level and cause of obstruction. Associated dilatation of the pancreatic duct and gallbladder are noted. Stones, masses, and strictures are searched for, as well as complications of these diseases: cholangitis, pancreatitis, stones, cirrhosis, liver abscess, and cholangiocarcinoma. If a malignant cause is found, suitability for resection is determined and staging is performed.
Radiography
Of all imaging modalities, endoscopic retrograde cholangiography (ERC) and percutaneous transhepatic cholangiography (PTC) can evaluate the bile ducts with the highest spatial resolution. They are, therefore, the gold standard for detecting stones, evaluating strictures, and localizing bile duct leaks. When the bile ducts are obstructed, the ducts closest to the site of injection are best demonstrated. ERC best evaluates the pancreaticobiliary junction and distal ducts, and PTC best evaluates the proximal intrahepatic ducts (see Figure 52-5 ). Two additional advantages of ERC are that the ampulla of Vater can be directly visualized and manometry can be performed. A potential pitfall of ERC is failure to recognize complete obstruction of a lobar or segmental bile duct. In this scenario, the obstructed ducts are absent from the cholangiogram because they are not opacified, and this absence may be difficult to appreciate, with the remaining ducts seeming complete.
ERC and PTC have a therapeutic as well as a diagnostic role. Removal of stones and sphincterotomy can be performed via ERC, external or internal-external drainage catheters can be inserted during PTC, and biliary dilation and insertion of stents can be performed by both approaches.
Endoscopic retrograde cholangiopancreatography (ERCP) has a complication rate of 1% to 5%. Moderate to severe pancreatitis is seen in 0.7%; the mortality rate associated with the procedure is approximately 0.2%.
Complications of PTC include cholangitis, sepsis, hemobilia, hemorrhage, and bile leak. The reported complication rate for PTC is approximately 2%. When percutaneous biliary drainage is performed, the complication rate rises to 5% to 7%. Factors that increase the likelihood of complications include poor preprocedure clinical status, coagulopathy, cholangitis, stones, and malignant, proximal, and multiple duct obstruction.
Cholangiography is highly sensitive for detecting bile duct stones and strictures. Stones are typically smooth filling defects. Other less common intraluminal filling defects include neoplasms (which can appear papillary, irregular, or smooth and demonstrate attachment to the bile duct wall), hemorrhage, sludge, and infected debris ( Figure 52-8 ).
Strictures may be due to a pathologic process of the bile duct wall and/or surrounding tissues. The cholangiogram should be interpreted in conjunction with cross-sectional imaging, because this provides complementary information, in particular, identification of a mass.
The classic cholangiographic features of a malignant stricture are irregular contour, abrupt transition (>1 cm), shouldered margins, asymmetry, and associated irregular or papillary intraluminal filling defects ( Figure 52-9 ). Benign strictures classically have a long transition and are smooth, gently tapered, and concentric ( Figure 52-10 ). Unfortunately, biliary strictures often are not classic in their appearance and the specificity of cholangiography for differentiating between benign and malignant disease is poor.
Computed Tomography
Multidetector computed tomography (MDCT) with intravenous contrast can produce high spatial resolution, with multiphase images that accurately assess the presence of biliary dilatation and detect and evaluate obstructing masses. The sensitivity of both CT and magnetic resonance imaging (MRI) for detection of pancreatic carcinoma is improved by scanning in the pancreatic phase. The pancreatic phase improves tumor conspicuity, maximizes the tumor-to-pancreas attenuation difference, and permits adequate arterial and mesenteric venous opacification for characterization of vascular invasion. The high spatial resolution of CT is particularly useful for local staging of malignancies such as pancreatic adenocarcinoma and cholangiocarcinoma ( Figure 52-11 ). CT or MRI can detect features that suggest a tumor is not suitable for resection; in cholangiocarcinoma, for example, these include hepatic parenchymal invasion; involvement of second-order bile duct branches in both lobes of the liver; vascular invasion or encasement of the proper hepatic artery, both right and left hepatic arteries, or main portal vein; extensive regional lymphadenopathy; and distant metastases.
CT without cholangiographic contrast is less sensitive than MRI for detection of choledocholithiasis. CT cholangiography using iodinated contrast agents such as intravenous meglumine iotroxate (which is taken up by the liver and excreted in bile) depicts bile duct stones more accurately. In one study, the sensitivity and specificity of CT cholangiography for detecting choledocholithiasis were, respectively, 87% and 96%, compared with 80% and 88% for magnetic resonance cholangiopancreatography (MRCP). However, several characteristics of cholangiographic contrast agents have limited their use: their biliary excretion is diminished in patients with elevated bilirubin levels or liver insufficiency, they can worsen renal impairment, and have a higher rate of adverse reactions compared with other iodinated contrast agents.
Dual-energy CT (DECT), an innovation that allows concurrent scanning with x-ray beams of different energies, is being explored for improved detection and characterization of pancreatic cancer. Virtual monoenergetic CT images and iodine-specific images generated after DECT acquisition have been shown to improve the conspicuity of isoattenuating pancreatic masses and depict small tumors that are difficult to identify on single-energy (120 kVp) CT images.
Magnetic Resonance Imaging and Magnetic Resonance Cholangiopancreatography
MRI, including MRCP, is extremely accurate in identifying the presence and level of biliary obstruction. It has a sensitivity of 92% for diagnosing stones and 88% for detecting malignant obstruction (with >90% specificity in each case).
MRCP does not require exogenous contrast agents. It uses heavily T2-weighted sequences to image bile and pancreatic secretions within their ducts. The most common sequences used are a multiple-section three-dimensional fast spin echo sequence with respiratory triggering and a single-section (thick slab) half-Fourier rapid acquisition with relaxation enhancement (RARE) sequence performed during a single breath-hold. Multiple-section acquisitions are usually postprocessed and displayed as maximum intensity projection (MIP), multiplanar reformatted images. These postprocessed images provide a useful overview of the pattern of biliary dilatation on one image. However, they may obscure small filling defects that are apparent on the thin-section source images ( Figure 52-12 ). Therefore, both source and postprocessed images should be reviewed. In addition, fluid within bowel can obscure the bile and pancreatic ducts on MIP and thick slab images, but this is rarely a problem on thin-section source images.
MRCP allows visualization of the bile ducts above and below the point of obstruction, a potential advantage over techniques using contrast (ERC and PTC). MRI is the most sensitive modality for the detection of a mass causing bile duct obstruction, owing to its superior contrast and temporal resolution. For instance, pancreatic adenocarcinoma is usually T1 hypointense, and fat-saturated T1-weighted images have been shown to improve its conspicuity. Pancreatic carcinoma is often well visualized as a hypointense mass in pancreatic phase postcontrast images, because it tends to be less vascular than normal glandular tissue and this phase provides superior tumor-to-gland attenuation difference ( Figure 52-13 ). Cholangiocarcinoma also may be hypointense on T-weighted imaging but characteristically shows delayed enhancement (at 1 to 5 minutes) because of intratumoral fibrosis, a feature that may be seen on MRI or CT ( Figure 52-14 ).
Depiction of the contour of strictures by MRCP is inferior to that with ERCP because MRCP has poorer spatial resolution.
The ampulla may be difficult to assess on MRCP for a variety of reasons, including interference from adjacent intraluminal gas, duodenal diverticula, duodenal wall contractions, and nondistention of the duodenal lumen. ERCP and endoscopic ultrasonography may image the distal CBD with greater resolution and accuracy.
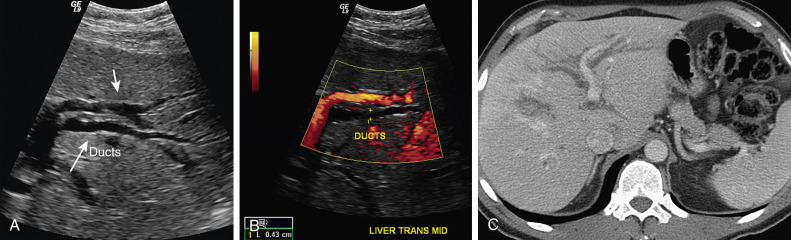
Ultrasonography
Transabdominal ultrasonography is often the initial imaging investigation for evaluating clinically suspected bile duct obstruction. It is readily available and sensitive for detecting biliary dilatation, gallbladder stones, and cholecystitis. It does not require contrast agents or prolonged patient immobility ( Figure 52-15 ). Overall, it is not as reliable as other modalities for determining the cause and level of bile duct obstruction. The main technical limitation is that bowel gas often obscures the distal CBD ( Figure 52-16 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







