Joseph M. Herman, Timothy M. Pawlik and Charles R. Thomas, Jr. (eds.)Medical RadiologyBiliary Tract and Gallbladder Cancer2nd ed. 2014A Multidisciplinary Approach10.1007/978-3-642-40558-7_23
© Springer-Verlag Berlin Heidelberg 2014
Future Directions
(1)
Department of Radiation Oncology and Molecular Radiation Sciences, Johns Hopkins University, Baltimore, MD, USA
(2)
Department of Radiation Oncology, Johns Hopkins University, Baltimore, MD, USA
(3)
Department of Surgery, Johns Hopkins Hospital, Baltimore, MD, USA
(4)
Department of Radiation Oncology, Oregon Health and Science University, Portland, OR, USA
Abstract
Improving the late detection and poor prognosis associated with gallbladder and biliary tract cancers remains a monumental task for physicians today. Recent developments in genome sequencing, pathology, functional imaging, and therapeutic approaches have resulted in enhanced diagnosis, staging, and management techniques of these cancers. However, to maximize the efficacy of these advancements, several considerations must be taken into account. Universal terminology should be defined because standard management guidelines cannot be established without uniform diagnosis and staging criteria. Multidisciplinary efforts among surgical oncology, medical oncology, radiation oncology, interventional radiology, pathology, and palliative care are fundamental to effective patient care as the collaboration of resources allows for more accurate treatment recommendations and favorable patient outcomes. Additional prospective clinical trials with large patient populations are needed to validate these findings and optimize full clinical potential in order to establish a standard of care for these patients.
Biliary tract malignancies consist of rare cancers that have detrimental impacts on the lives of their victims. The first report of biliary tract carcinoma dates back to the 1840 [1], prior to the modern era of scientific discoveries and technological innovations. Despite advancements in medicine and our recognition of biliary tract cancers for centuries, biliary tract carcinomas are frequently associated with late detection and poor prognosis. Incidence rates of biliary cancers in the United States have doubled within the past 10 years, with an increase of 7,100 diagnoses in 2002 to a projection of approximately 14,000 new cases in the year 2013 [2, 3].
Due to the rare origin and anatomical, histological, and biological heterogeneity of biliary tract and gallbladder cancers, scientific discovery in this field is fairly scarce. Although known risk factors exist, patients with these cancers are commonly asymptomatic, consequently causing them to present with advanced disease. Accurate classification and differentiation of morphological and histological tissue are essential to determine the exact incidence and risk factors of these cancers. No standard of care treatment for biliary tract carcinomas has been accepted, and large clinical trials are required to optimize full clinical potential to make historic advances in this field of study.
Fortunately, however, with increased incidence comes increased technology. Current findings have made an immense contribution to the diagnosis and management of biliary tract cancers; from herbal medicine to exome sequencing, innovative ideas are becoming reality. The latest discoveries are discussed here to suggest what lies ahead in the future efforts to improve outcomes of patients with biliary tract and gallbladder cancers.
1 Genome Sequencing
Recent advances in cancer genomics have been significant in identifying biological processes to improve prognostication and evidence-based management to ultimately lead to personalized medicine. Prevention of tumor formation and early diagnosis is difficult with biliary tract carcinomas due to their poor sensitivity to conventional therapies. Therefore, progressive efforts have been made to understand the underlying cellular mechanisms among abnormal cells and to develop innovative therapies accordingly. Technological advances in molecular profiling have conveyed common molecular deviations in biliary tract cancers, allowing for a better understanding of the biology of the cancer and possible drug therapy.
Biliary tract cancers arise from the epithelial lining, due to overexpression of epidermal growth factors or mutated signaling pathways. Precise clarification of these cellular pathways, in accordance with the advancement of technology, is paramount to developing efficient expansion in molecular profiling techniques. Several complex technologies are being used to complete mutation analysis of biliary tract cancers including mass spectrometry, SNaPshot genotyping, and whole exome sequencing [4]. Recent analysis of recurrent mutations in routine diagnostic pathology specimens, using SNaPshot technology, revealed a distinct signature involving IDH1/2 gene mutation in intrahepatic cholangiocarcinoma, suggesting a possible therapeutic target in the future [5]. Additionally, a new computational technology called heterogeneous expression profile analysis (HEPA) has been developed to highlight specific genes that are heterogeneously overexpressed by cancer cells in comparison with their normal counterparts using an innovative mathematical scheme to prioritize clinically relevant tumor-specific antigens [6].
The heterogenic nature of biliary tract malignancies signifies more intricate subtyping, therefore requiring personalized tumor profiling and treatment therapy [7]. The introduction of molecular-targeted therapy for biliary tract carcinomas has increased our understanding of the molecular behavior of tumors in order to introduce novel therapeutic agents. There are two possible options in using molecular targeting agents—as first-line therapy used simultaneously with standard chemotherapy or as second-line therapy alone. Currently, there are limited findings on molecular–biological characteristics of biliary tract carcinomas, but further research on EGFR and anti-angiogenic inhibitors such as erlotinib, lapatinib, and sorafenib is soon expected [8]. Recently discovered pathways and/or mutations where molecular targeting agents could be effective include the PIK3CA/AKT/mTOR pathway, MET receptor tyrosine kinase inhibitors, ROS-1 translocations, and BRAF mutations [7]. Additionally, current studies report the significant role that zinc finger X-chromosomal protein (ZFX) has in gallbladder cancer cell proliferation and migration, which could potentially lead to therapies targeting the transcription factor [9]. With the rapid evolution of genome technology, the technical and economic feasibility of complete molecular profiling of a patient’s tumor has increased substantially [10].
2 Diagnostic and Staging Technique
2.1 Pathology
Specific dissection of the histopathology and pathogenesis of bile tract carcinomas must be undertaken to advance current diagnostic, prognostic, and staging efforts. Complete characterization of the pathogenesis and prediction of metastatic involvement are of utmost concern in regard to the nature of these heterogeneous malignant cells. Can routine biopsies prevent advanced disease? Recently, the role of laparoscopic interaortocaval (16b1) lymph node biopsy and frozen-section histopathological examination in gallbladder cancer is being studied to determine the potential effects on surgical management [11]. Studies of pathogenesis also reveal the possible role of hepatic progenitor cells in the malignant progression of intrahepatic cholangiocarcinoma, inherently adding clarification to the heterogeneity of such tumors [12]. Additionally, there is supporting data illustrating a direct correlation between microRNA (miRNA) and hepatobiliary neoplasia, which can lead to potential diagnostic biomarkers and new miRNA-based therapies in biliary tract cancers [13].
Because of the heterogeneity of biliary tract and gallbladder carcinomas, many biomarkers are expected to exist. For example, nemo-like kinase (NLK) has recently been associated with advanced tumor progression and poor clinical outcome in gallbladder cancer patients, which provides support of its potential to be used as a prognostic marker of gallbladder cancer [14]. Proteomic analysis of biliary carcinomas frequently serves as an approach to discover new biomarkers. According to Mulvenna et al. [15], cutting-edge technology in proteomics such as laser capture microdissection (LCM), isotopic labeling, sophisticated hypothesis-driven targeted mass spectrometric analyses, and the combination of these technologies can be used to detect and identify novel protein biomarkers. One such proteomic technique used recently was matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) combined with weak cation-exchange (WCX) magnetic beads and pattern recognition to rapidly detect cancer-related proteins [16]. Additionally, a pursuit for a serological method to noninvasively diagnose cholangiocarcinoma has been put forth. WFA-positive L1CAM has been found to be a highly specific cholangiocarcinoma marker in bile and serum, and further investigation is being explored to enhance the reliability of the present system [17].
2.2 Imaging
Within the past few decades, the introduction of advanced image-guided diagnostic tools has made a large impact on the diagnosis and treatment of biliary tract and gallbladder carcinomas. Ultrasonography (US), computed tomography (CT), positron emission tomography (PET), magnetic resonance imaging (MRI), and cholangiography are beneficial imaging modalities currently being used. However, each modality has its advantages and disadvantages, and continued investigation is necessary to discover the most efficient diagnostic and staging techniques.
Most often, contrast-enhanced and endoscopic US are used in the early detection of bile duct tumors. A modern investigation confirmed that endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is highly specific (100 %) with sensitivity rates of 87 and 80 % in the diagnosis of biliary tract and gallbladder cancer, respectively [18]. EUS-FNA has also recently been shown to be more accurate than CT or PET for the evaluation of regional lymph node metastases in patients with cholangiocarcinoma [19]. Additional studies are seeking the potential usefulness of new contrast agents in endoscopic and contrast-enhanced US. Finally, recent advances in contrast-enhanced US are expected to increase capabilities for early diagnosis.
A recent multicenter study investigated the usefulness of contrast-enhanced ultrasound (CEUS) in the diagnosis of gallbladder carcinoma, and ultimately concluded that CEUS is undoubtedly superior to other ultrasound techniques in distinguishing biliary sludge from gallbladder lesions [20]. Additionally, Doppler and color Doppler ultrasound have been identified as beneficial to assessing vascularity of hepatobiliary lesions and vascular anatomy [21] as well as indicating expansive lesions by ruling out biliary sludge and cholesterol polyps [22]. However, more investigation into the imaging modality of ultrasound is essential. Despite the numerous advancements in ultrasound imaging, the majority of gallbladder masses identified preoperatively have been found to be “pseudo-masses” on final pathology [23]. Further investigation seeking high frequency probes [24], color Doppler, and contrast agents will dramatically enhance the effectiveness of ultrasound imaging.
In recent years, state-of-the-art cross-sectional imaging such as CT, MRI, and magnetic cholangiopancreatography (MRCP) has provided high-resolution imaging in contribution to the planning and treatment process of biliary tract cancers. Until recently, endoscopic retrograde cholangiopancreatography (ERCP) has been the gold standard in diagnosing pancreaticobiliary malignancies, though recent findings suggest MRCP is a more effective noninvasive method for obtaining quality images of the biliary tract [25]. Additionally, recent advances in multidetector row-computed tomography (MDCT) have demonstrated increased accuracy rates of differential diagnosis of gallbladder polyps [26]. MRI modifications such as an increased flip angle have resulted in significantly enhanced visualization of the biliary tree [27]. Newly improved instrumentation of MRI has greatly increased spatial and contrast resolution as well as signal-to-noise ratio [26] although improvements of more intervention-compatible technology would have great clinical potential.
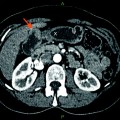
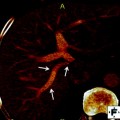
Simultaneous PET/CT imaging has great potential in the staging, restaging, and determination of recurrence in biliary tract cancers although current studies are in search of alternate PET radiotracers to 18F-FDG that are tumor-specific. For example, 68Ga-NOTA-RGD has been recently discovered as a PET radiotracer to visualize angiogenesis and is a promising candidate for cancer imaging [28]. Very recently, however, combined PET/MRI has become increasingly popular. PET/MRI is believed to effectively integrate individual molecular, functional, and anatomical imaging techniques to be used in radiotherapy treatment planning [29]. Although current research on the advantage of PET/MRI in biliary tract tumors is limited, separately acquired data from PET/CT and MRI fusion images suggest that PET/MRI has an advantage over PET/CT [30




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree