Meckel’s Diverticulum
Clinical Findings
Meckel’s diverticulum is a common congenital abnormality of the gastrointestinal tract that can produce varied complications and therefore diverse clinical manifestations. It is the most frequent in a spectrum of abnormalities that occur in the small bowel secondary to failed regression in early fetal life of the omphalomesenteric (vitelline) duct, the tract through which the primitive midgut communicates with the yolk sac. Incomplete resorption of the duct can produce abnormalities anywhere along its course between the ileum and the umbilicus, which in addition to Meckel’s diverticulum include omphalomesenteric or mesodiverticular bands, vitelline fistula, omphalomesenteric cyst, and umbilical polyps ( Fig. 117-1 ).
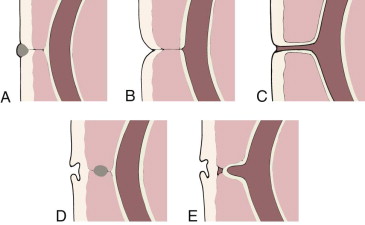
Meckel’s diverticulum is a result of the patency of the ileal end and closure of the umbilical end of the omphalomesenteric duct and is a true diverticulum, composed of all bowel layers. Unlike alimentary duplications and most other bowel diverticula, Meckel’s diverticulum arises from the antimesenteric border of the bowel and has a separate blood supply, the vitellointestinal artery. According to the “rule of 2’s,” Meckel’s diverticulum has an incidence of approximately 2%, arises in the ileum within 2 feet of the ileocecal valve, and becomes clinically symptomatic by the age of 2 years.
Clinical symptoms secondary to complications of Meckel’s diverticulum are varied and occur in approximately 4% of patients with Meckel’s diverticulum. The most common complications are rectal hemorrhage and intussusception.
Painless lower gastrointestinal bleeding is a major complication of the approximately 20% to 55% of Meckel’s diverticula that contain acid-secreting ectopic gastric mucosa and is more frequent in children than in adults. The small bowel adjacent to the ectopic gastric mucosa becomes ulcerated from exposure to the acid, resulting in hemorrhage. Ileoileal and ileocolic intussusceptions result when Meckel’s diverticulum serves as a lead point, and an irreducible intussusception should raise suspicion for Meckel’s diverticulum. Other less common complications of Meckel’s diverticulum include small bowel obstruction from volvulus or hernia around an associated omphalomesenteric band; bowel perforation; diverticulitis or inflammation of the diverticulum itself; and Littre hernia, which is an inguinal hernia containing Meckel’s diverticulum.
A specific form of Meckel’s diverticulum is giant Meckel’s diverticulum, which tends to be larger than the average size of 2 × 3 cm and is long with a narrow neck. It can serve as a lead point for focal volvulus or may undergo torsion at its base. Stasis in enormous Meckel’s diverticula may lead to bacterial overgrowth, resulting in malabsorption.
Imaging Findings
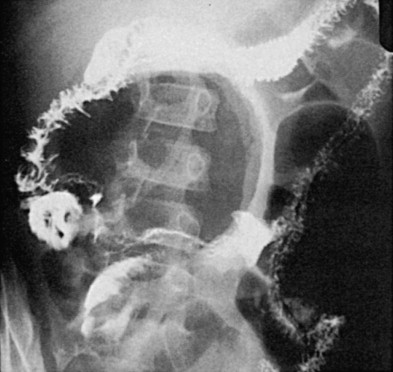
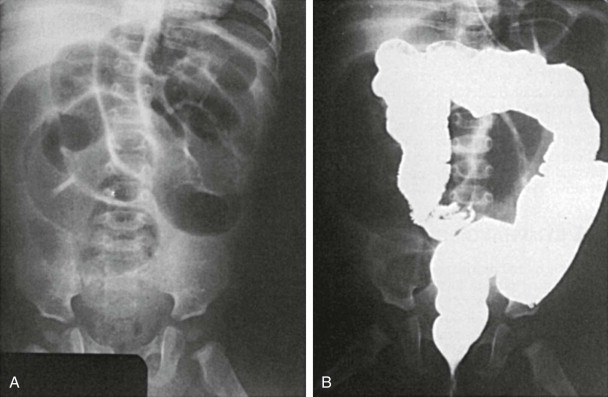
Plain abdominal radiographs usually have normal findings but may show obstruction if the diverticulum is complicated by intussusception. Meckel’s diverticulum rarely fills on routine barium studies, possibly because of small size or blockage of the lumen with ingested debris, but it may opacify with the higher pressures of enteroclysis. Difficulty in detection is compounded by overlying bowel loops that may obscure the diverticulum unless they are displaced with compression. A mucosal triangular plateau or a triradiate fold pattern in the right lower quadrant has been described in small bowel examinations in patients with Meckel’s diverticulum. Air-filled giant Meckel’s diverticula may fill with contrast medium on delayed studies ( Fig. 117-2 ). Children with small bowel hernia or volvulus around an omphalomesenteric band often show nonspecific findings of lower small bowel obstruction ( Fig. 117-3 ).
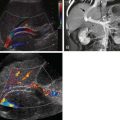
Advanced imaging including computed tomography (CT) and ultrasound is routinely used to study children who present with symptoms of an acute abdomen, especially right lower quadrant pain. Meckel’s diverticulum itself as well as secondary complications such as intussusceptions can be detected ( Fig. 117-4 ). On sonography, inflamed Meckel’s diverticulum can appear as a cystic or tubular structure with thickened walls, sometimes displaying the characteristic alternating hyperechoic and hypoechoic bands of intestinal wall. Doppler and color Doppler sonography may demonstrate the inflammatory changes more clearly. By CT or magnetic resonance (MR) enterography, the inflamed diverticulum may appear as an air-, fluid- or contrast-filled blind-ending pouch with thickened walls in the right lower quadrant with inflammatory changes in the adjacent mesentery. Inverted Meckel’s diverticulum, often the lead point of an intussusception, may also be seen as a filling defect in the opacified lumen.
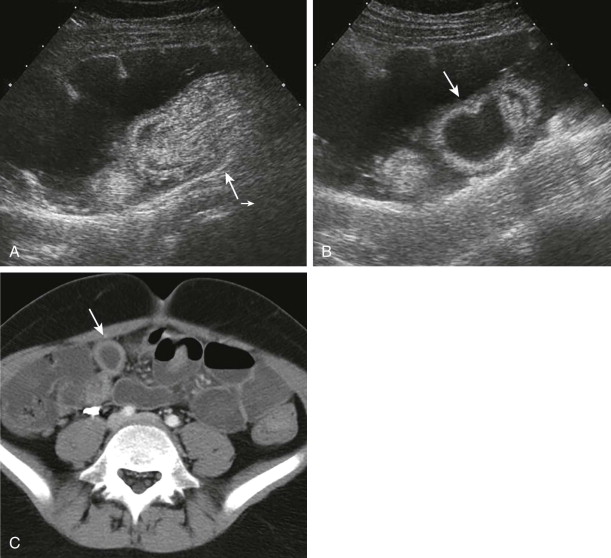
Dilated and inflamed Meckel’s diverticulum can resemble the dilated and inflamed appendix in appendicitis by sonography and CT. Clinical symptoms of right lower quadrant pain and fever also overlap in the two entities, and identification of the normal appendix in cases of suspected Meckel’s diverticulum is recommended to aid in differentiation between the two. An ileal duplication cyst may mimic Meckel’s diverticulum; Meckel’s diverticulum is distinguishable from an ileal duplication cyst by its thicker, more irregular wall and the presence of peristalsis.
Technetium Tc 99m pertechnetate nuclear scintigraphy is the most widely used method for diagnosis of bleeding Meckel’s diverticula, and it has a sensitivity of 85%. The intravenously injected isotope localizes in the right lower quadrant of the abdomen within the ectopic gastric mucosa, with rate and pattern of activity mirroring that of gastric mucosa in the stomach ( Fig. 117-5 ). Administration of pentagastrin before scintigraphy may be used to stimulate diverticular gastric mucosal uptake and thereby enhance sensitivity of detection if the result of a prior study has been negative or equivocal and Meckel’s diverticulum is still strongly suspected.
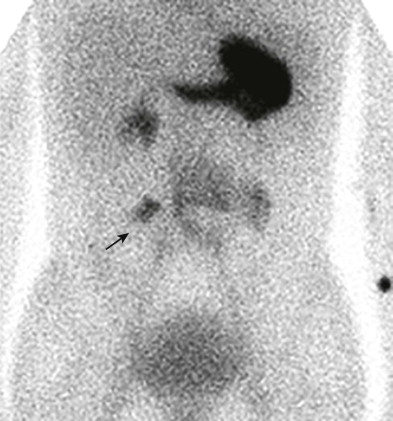
False-negative study results can occur when residual gastrointestinal barium absorbs the emitted gamma rays, when profound ulceration or infarction has destroyed the gastric mucosa within the diverticulum, or when the isotope is incorrectly interpreted as being within the genitourinary tract. Lateral scans of the abdomen are performed routinely to minimize the last error. False-positive study results can also occur, but they are easier to recognize. Ectopic gastric mucosa in alimentary cysts is responsible for most of the false-positive results. Inflammatory processes may rarely localize the isotope, despite the absence of gastric mucosa.
Intestinal Lymphangiectasia
Clinical Findings
Congenital and acquired disorders of the small bowel lymphatics can produce protein loss, diarrhea, and decreased immunoglobulin levels. Histologic changes include diffuse or focal dilation of lymphatics in all bowel layers that may be accompanied by villous changes and infiltration of the mucosa by inflammatory cells. Leakage of protein-rich lymphatic fluid from the dilated lymphatic channels into the gastrointestinal tract results in protein-losing enteropathy. Congenital lymphatic structural malformation is termed primary intestinal lymphangiectasia (Waldmann’s disease) and typically is manifested before 3 years of age. Common symptoms include bilateral lower extremity edema and gastrointestinal complaints such as diarrhea, abdominal pain, nausea, and vomiting.
Because of the protein loss through the small bowel, the child may fail to thrive. Similar loss of lymph cells may produce lymphopenia. Definitive diagnosis of primary lymphangiectasia is made by endoscopy and intestinal biopsy. Elevated levels of α 1 -antitrypsin in a 24-hour stool sample are indicative of protein-losing enteropathy and support the diagnosis.
When dilation of lymphatic channels is secondary to venous obstruction or elevated venous pressure, the disorder is termed secondary lymphangiectasia. Conditions known to cause secondary lymphangiectasia include inflammatory bowel disease, sarcoidosis, lymphoma, congestive heart failure, and constrictive pericarditis. Secondary lymphangiectasia is an uncommon but well-recognized complication in children who have undergone a Fontan procedure for complex congenital heart disease. Intestinal lymphangiectasia has long been recognized as part of the Noonan, Turner, Klippel-Trénaunay, and von Recklinghausen syndromes. Hennekam syndrome, an autosomal recessive disorder with mild to moderate mental retardation, peculiar facies, and ear defects, is also associated with lymphangiectasia.
Imaging Findings
Small bowel series show thickening of the valvulae conniventes, nodularity of the mucosa, and excess secretions if there is malabsorption. The caliber of the gut is normal. Barium enema examination may show thickening of affected colonic folds.
Sonography and CT can also demonstrate the nonspecific findings of ascites, dilated lymphatics, and thickened bowel walls and mesentery. The last changes may be primary or result from the hypoproteinemia caused by this protein-losing enteropathy.
Functional imaging with human serum albumin nuclear medicine scintigraphy plays an important role in the work-up of suspected intestinal lymphangiectasia and has the advantage over α 1 -antitrypsin stool sampling of the ability not only to confirm the presence of the protein loss from the gastrointestinal tract but also to localize the anatomic site of protein leakage. In this diagnostic procedure, 99m Tc-labeled human serum albumin is injected intravenously, and periodic images of the abdomen are obtained during a 24-hour period in search of abnormal radiotracer activity seeping in the bowel. Other radiopharmaceuticals used for this purpose include 99m Tc–methylene diphosphonate and 99m Tc-dextran.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree