Chapter Outline
Splenic Histology and Function
Splenic Histology and Function
Splenic tissue is composed of red pulp and white pulp. The red pulp is composed of vascular sinusoids, and the white pulp is composed of lymphoid follicles with white blood cells. The proportion of white pulp relative to red pulp increases with age and antigenic stimulation. Mononuclear phagocytic cells of the red pulp remove abnormal or senescent red blood cells from circulation.
The radiographic appearance of the spleen depends on the imaging modality, age of the patient, splenic composition and size, and timing of intravenous administration of contrast agents. The spleen has the shape of a curved wedge and may have normal clefts, notches, or lobules.
On ultrasound, the normal spleen has a homogeneous echotexture that is hyperechoic to the pediatric kidney and isoechoic or hyperechoic to the liver on gray-scale images ( Figs. 122-1 and 122-2 ). The parenchyma is exceptionally vascular on color flow Doppler imaging.


Precontrast computed tomography (CT) examination of the normal spleen demonstrates uniform parenchymal attenuation that is slightly less than that of the normal liver. On contrast-enhanced examinations, the spleen’s appearance depends on the timing of the intravenous administration of the contrast bolus and image acquisition. Transient heterogeneous enhancement of the spleen is presumed to result from differences in relative flow rates through the red and white pulp. The mean time of initial visualization of the heterogeneity after infusion of the contrast agent is 19.2 seconds (range, 9-44 seconds). Normal transient heterogeneity persists 70 seconds after administration of the contrast agent in only 6% of children. The patterns as seen on contrast-enhanced CT and magnetic resonance imaging (MRI) examinations are varied and include arcs, stripes ( Fig. 122-3 ), and focal areas of relatively delayed perfusion. Heterogeneous areas in the spleen on images acquired more than 70 seconds after the administration of contrast material may indicate an abnormality.

The tissue characteristics related to the ratio of red and white pulp probably dictate the signal intensity on MRI. In the neonate, the lymphoid tissue of the white pulp is not well developed, and the spleen is composed primarily of the vascular sinusoids of the red pulp. In the first week of life, the spleen usually is isointense or hypointense relative to the liver on T1- and T2-weighted, spin-echo images ( Fig. 122-4A, B ). With the maturation of high cellular water content lymphoid tissue, the spleen becomes minimally hyperintense relative to the liver on T2-weighted images at approximately the second week of life. When the child is 1 month old, the spleen is moderately hypointense relative to the liver. There is a relative increase in the size and number of lymphoid follicles and a relative decrease in the extent of red pulp up to the age of 7 months ( Fig. 122-4C, D ). After the child is 8 months old, the signal characteristics of the pediatric spleen are the same as in the adult. As on contrast-enhanced CT, the spleen often shows heterogeneous enhancement during the arterial phase of dynamic contrast-enhanced MRI. It has been theorized that the decreased signal intensity of the spleen relative to the liver in patients after chemotherapy, with or without associated blood transfusions, may result from reduction or elimination of the white pulp’s high water content and lymphoid tissue.

Nuclear medicine studies to evaluate the spleen include technetium 99m Tc sulfur colloid scintigraphy and 99m Tc-denatured (heat-damaged) red cell scintigraphy. Sulfur colloid is phagocytosed by the reticuloendothelial cells of the spleen and liver, and denatured red cells are sequestered in the spleen. Indications for splenic scintigraphy include evaluation of abdominal trauma, splenomegaly, and left upper quadrant mass; search for accessory spleens or splenosis; and assessment of infarction, functional asplenia, polysplenia, and the heterotaxy syndrome.
Spleen Size
The spleen increases in length and volume as the child grows. Splenic length is typically measured sonographically in the coronal plane through the hilum, from the dome of the spleen to the inferior tip. Upper limits of normal for age are 6 cm at 3 months, 6.5 cm at 6 months, 7 cm at 12 months, 8 cm at 2 years, 9 cm at 4 years, 9.5 cm at 6 years, 10 cm at 8 years, 11 cm at 10 years, 11.5 cm at 12 years, and 12 cm at and beyond 15 years for girls and 13 cm for boys 15 years and older. Secondary imaging signs of splenomegaly include extension of the spleen below the inferior margin of the left kidney or the right hepatic lobe, medial extension to the aorta, and loss of the concavity of the hilum ( Fig. 122-5 ).

Congenital Abnormalities
Splenules
Splenules are congenital rests of normal splenic tissue that form when mesenchymal cells fail to fuse with the rest of the splenic mesenchyme. A splenule is also known as an accessory spleen, supernumerary spleen, or splenunculus. Splenules are typically located near the main splenic hilum anteriorly or posteriorly but are rarely lateral to the main spleen and have not been documented superior to the main spleen.
Splenules are circumscribed, round, oval, or triangular, and they are homogeneous with and without enhancement ( Fig. 122-6 ). Splenules vary in size from millimeters to centimeters, usually less than 3.2 cm in diameter, and are capable of significant hypertrophy.

Splenic artery supply to a splenule may be visible on CT. On CT, 16% of people have one to three accessory spleens, and the presence of 10 splenules has been described. Splenules are seen in 10% to 30% of people at surgery or autopsy. Subcentimeter splenules may have lower attenuation than the main spleen because of volume averaging with surrounding fat and the CT collimation used.
Splenules need to be removed if the patient is undergoing therapeutic splenectomy for a hematologic disease because the residual splenic tissue can be responsible for recurrent disease. Accessory spleens may resemble lymphadenopathy or neoplasm if they are situated near the greater curve of the stomach, in the left adrenal, or within the pancreatic tail. Splenules may become symptomatic if they twist.
Polysplenia and Asplenia
Multiple spleens (i.e., polysplenia) or absence of the spleen (i.e., asplenia) at birth can be associated with multiple congenital anomalies under the heading of heterotaxy or situs ambiguus. In polysplenia, multiple small spleens are always seen on the same side as the stomach because of the origin of splenic tissue from the dorsal mesogastrium ( Fig. 122-7 ). Polysplenia is often associated with an interrupted inferior vena cava with azygos continuation, bilateral left-sidedness, and congenital heart disease. There may also be associated absence of the gallbladder, biliary atresia, preduodenal portal vein, and malrotation of the bowel.

Asplenia is the absence of splenic tissue and is associated with bilateral right-sidedness and complex cyanotic heart disease. There is an association with gallbladder duplication, midgut malfixation or malrotation, and microgastria.
Nuclear scintigraphy with 99m Tc-sulfur colloid or heat-damaged 99m Tc-labeled erythrocytes can evaluate for the presence or absence of splenic tissue.
Wandering Spleen
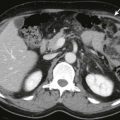
The spleen is primarily supported by the gastrosplenic and splenorenal ligaments. If the support ligaments are absent or lax, the spleen may be ectopic from its normal position and is referred to as a wandering spleen. It is rare in children. The degree of ectopia is limited by the length of the vascular pedicle. The absence of the spleen in its usual position and presence of a soft tissue mass elsewhere with the imaging characteristics of the spleen characterize this entity ( Fig. 122-8 ). There may be a history of intermittent abdominal pain or an intermittently palpable abdominal mass. The child with torsion of a wandering spleen usually presents with an acute surgical abdomen.

Ultrasound evaluation of torsion of the wandering spleen may demonstrate areas of hyperechoic hemorrhage and hypoechoic infarction and congestion. Early, the gray-scale echotexture may be preserved with or without splenomegaly, and color Doppler examination may demonstrate splenic vein occlusion or stasis with preserved arterial flow and intraparenchymal flow. As the torsion persists, arterial inflow eventually decreases or ceases to be detectable.
On CT, the spleen with torsion may be heterogeneously enhancing and have decreased attenuation, depending on the extent of perfusion. The enhancing hilar vessels twist with the interpositional hilar fat and yield a whorled or banded appearance ( Fig. 122-9 ). On ultrasound examination, twisted hilar vessels appear as a splenic hilar mass, which may be an important finding for cases in which the diagnosis is not yet made and the twisted spleen is not ectopic. The wandering spleen is associated with deficiency of anterior abdominal wall musculature and is rarely reported with prune-belly syndrome.

After diagnosis of torsion of a wandering spleen, if the spleen is not infarcted, splenopexy may be performed in an effort to preserve splenic function by preventing future torsion.
Splenogonadal Fusion
The splenic anlage and ipsilateral gonadal anlage have a close developmental relationship and may fuse, forming a long, continuous cord from the gonad cephalad to the main spleen. The connection to the main spleen may be totally or partially fibrous and is associated with cryptorchidism. Alternatively, a portion of the splenic tissue may separate from the main spleen ( discontinuous ) and descend with the gonad, known as splenogonadal fusion.
Male children with splenogonadal fusion typically present with a painless testicular mass. Splenogonadal fusion should be considered preoperatively in evaluation of a scrotal mass because failure to do so may result in unnecessary orchiectomy. The ectopic splenic tissue attached to the testicle mimics testicular duplication on ultrasound and has been described as an encapsulated, homogeneous extratesticular mass that is isoechoic with the normal testis and shows no hyperemia on color Doppler ultrasound. The presence of ectopic splenic tissue can be confirmed with nuclear 99m Tc-sulfur colloid scintigraphy.
Cystic Lesions
True Cysts and Pseudocysts
True cysts, such as congenital or epithelial cysts and echinococcal cysts, have an epithelial cell lining, but pseudocysts do not. It is not possible to reliably differentiate between true cysts and pseudocysts by imaging.
Epithelial cysts can be further categorized as mesothelial, epidermoid, or dermoid. Epidermoid cysts are lined by stratified squamous epithelium, which macroscopically appears coarsely trabeculated. Epidermoid cysts may be familial, are often several centimeters in diameter, and have a tendency to rupture.
Epidermoid cysts on ultrasound appear as relatively thin walled, anechoic lesions that do not change over time. Mural calcification is seen in 10% of cases, although it is seen more often in acquired pseudocysts. There may be septations and wall trabeculation, which are seen more often in true cysts. The fluid may have some internal echoes caused by cholesterol crystals, inflammatory debris, or blood products from prior hemorrhage. On CT and MRI, cysts follow fluid attenuation and signal character, with or without septations and calcification ( Fig. 122-10 ). These cysts show no central or rim enhancement.

Echinococcal cysts are true cysts. They can have peripheral calcification and often appear to have multiple septa. In echinococcal cysts, there may be internal areas of increased attenuation on CT due to dense debris, also known as hydatid sand. Peripherally located small daughter cysts may be present.
Acquired pseudocysts, most often post-traumatic, are more common than true cysts. They are indistinguishable on imaging from true cysts and may have mural calcification, but septa are uncommon.
Lymphatic Malformations
Lymphatic malformations of the spleen, also known as lymphangiomas, are congenital and are composed of dilated lymphatic channels. Despite their rarity in the spleen, lymphatic malformations are the second most common benign splenic lesion. They are not neoplastic, and their growth is commensurate with that of the child. An abrupt change in lymphatic pressure or flow, infection, or hemorrhage into the lesion can cause an abrupt increase in size. They are frequently asymptomatic and are found incidentally ( Fig. 122-11 ).

The lymphatic channels and compartments are lined by a single layer of flattened endothelial cells and contain lymphatic fluid. They can be solitary, or they may be associated with other lymphatic malformations of the viscera, bone, or soft tissues.
On imaging, lymphatic malformations of the spleen appear similar to lymphatic malformations elsewhere in the body, with a multiloculated, cystic configuration. On ultrasound, there are well-defined, hypoechoic or anechoic spaces of various sizes, typically with septations and proteinaceous debris that may produce internal echoes. Radionuclide 99m Tc-sulfur colloid scans show a focal area of decreased uptake.
On CT, lymphatic malformations are hypodense with nonenhancing discrete areas that are often subcapsular, but they can be diffuse. The walls and septa may faintly enhance after administration of contrast material.
On MRI, the cystic areas are usually hypointense on T1-weighted images and hyperintense on T2-weighted images. They may appear hyperintense on T1-weighted images because of proteinaceous contents or prior hemorrhage, with or without fluid-fluid levels on T2-weighted sequences. The cystic areas do not enhance, but the septa may enhance on the delayed phase images.
Benign Lesions
Hemangiomas
Before the classification by Mulliken and Glowacki, hemangiomas and vascular malformations had variable nomenclature in the literature, leading to classification confusion. The International Society for the Study of Vascular Anomalies (ISSVA) has subsequently established guidelines based on the histopathology, clinical course, and treatment of these lesions. Despite the criteria outlined in the ISSVA guidelines, lesions referred to as hemangiomas are the most common benign lesions of the spleen.
The imaging appearance of pediatric splenic hemangiomas on ultrasound, CT, and MRI is variable, as reported in the literature. This probably reflects the widespread use of inaccurate terminology in the medical literature, as some authors were likely describing vascular neoplasms, whereas others were describing vascular malformations.
Hemangiomas are benign and usually asymptomatic solid neoplasms composed predominantly of a mass of endothelial cells. Proliferating hemangiomas may cause clinical complications by location, mass effect, or platelet sequestration. They have an initial proliferative phase during the first year of life, followed by an involution phase. In the proliferative phase, a hemangioma is seen on MRI as a solid, lobulated, soft tissue mass that is isointense or hypointense to muscle on T1-weighted images and hyperintense on T2-weighted images, with uniform contrast enhancement. Feeding arteries and draining veins may be seen and can demonstrate prominent flow voids. During involution, there is fatty replacement of the neoplasm. There is a variable increase in signal on T1-weighted images and decreased signal on T2-weighted images.
Vascular Malformations
Vascular malformations are congenital lesions composed of some combination of arterial, capillary, venous, and lymphatic channels. They are described by their flow characteristics (i.e., high or low flow). Venous malformations are low-flow vascular malformations that were formerly known as cavernous hemangiomas, varicose hemangiomas, or lymphangiohemangiomas. Vascular malformations are distinguished from vascular neoplasms, such as hemangiomas, by the lack of increased endothelial cell turnover. They are composed of endothelial cell–lined channels, with interconnecting venous spaces and various connections to normal or dysplastic draining veins. The spaces are filled with blood, and there is no true solid mass. They may contain phleboliths. They have low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. The walls of the vascular spaces may appear as septations that do not enhance, whereas the vascular spaces do enhance if they are not thrombosed. Fluid-fluid levels may be seen. Phleboliths appear as signal voids on MRI.
Hamartoma
Hamartomas are rare, non-neoplastic, disorganized mixtures of normal splenic components. They usually are not encapsulated but are well-circumscribed, solid, nodular lesions. They often appear hyperechoic and may have cystic areas within them. Some are heterogeneous and may contain minute, speckled calcifications or a central, stellate scar. Hamartomas range in size from a few millimeters to centimeters, with a median size of 5 cm.
Almost half of children with splenic hamartomas are symptomatic at presentation. Children may present with splenomegaly, recurrent infections, periodic low-grade fever with night sweats, growth retardation, or hematologic abnormalities.
Nuclear scintigraphy may demonstrate splenomegaly without a shift in radionuclide uptake. The cross-sectional imaging appearance and degree of contrast enhancement vary. CT may demonstrate splenomegaly with or without a contour abnormality. On MRI, splenic hamartomas are often isointense with normal spleen on T1-weighted images and have variable signal intensity on T2-weighted images. There is diffuse heterogeneous early enhancement after administration of contrast material and more uniform, prolonged enhancement on delayed images, except in cystic areas or central scar. MR characteristics of the mass, combined with uptake of 99m Tc-sulfur colloid, are most suggestive of a benign hamartoma. Splenic hamartomas have been reported in association with tuberous sclerosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree