Congenital Anomalies
Most anomalies that affect the stomach and duodenum are evident on plain radiographs of the abdomen. Complete atresia of the pylorus results in a single bubble of air, atresia of the duodenum is manifested as the well-known radiographic double bubble, and duplication cysts can displace or obstruct bowel. Microgastria results in absence of the normal gastric bubble and is associated with cardiovascular and splenic anomalies. When an anomaly of stomach or duodenum is encountered, careful search for other anomalies is mandatory.
Pyloric and Antral Atresias
Pyloric and antral atresias are rare anomalies in which the neonate is unable to feed without vomiting. The abdomen is gasless except for the stomach bubble. Sonograms performed on infants with pyloric atresia show neither a normal canal nor muscle of the pylorus. A pyloric channel should be apparent in babies who have a complete pyloric membrane. The rare neonate who has pyloric stenosis has incomplete obstruction and typical features of pyloric stenosis. Differentiation between atresia and membrane usually rests with the surgeon because plain radiographs showing complete obstruction in the first day of life mandate an operation. There is an association between pyloric atresia and epidermolysis bullosa. In affected patients, minimal trauma to the skin results in blisters and erosions. Pyloric obstruction in these patients may begin in utero or develop postnatally.
Antral Mucosal Diaphragm
Complete mucosal diaphragm or web of the antrum is an uncommon anomaly that may cause vomiting or failure to thrive in infants. Older children may present because of gastric retention of a coin or other foreign body. On upper gastrointestinal studies, the thin membrane is manifested as a linear filling defect at the level of the antrum ( Fig. 116-1 ). In symptomatic infants, the aperture of the diaphragm is often 5 mm in diameter or less, although the size of the aperture cannot be judged accurately on fluoroscopic examination. Asymptomatic, incidentally discovered webs probably do not require intervention, although some physicians advocate a more aggressive approach.

Microgastria
Microgastria is often associated with absence of the spleen and mesenteric abnormalities. An association with laryngotracheoesophageal clefts, tracheoesophageal fistula, and the VACTERL (vertebral, anorectal, cardiac, tracheoesophageal, renal, and limb) spectrum of anomalies has been documented. In those patients with isolated microgastria, plain radiographs show no normal gastric air bubble and the esophagus is often dilated because it functions as an accessory stomach through reflux. The miniature stomach empties into a duodenum of normal caliber, which may be in an abnormal position ( Fig. 116-2 ). Treatment is directed toward increasing gastric capacity by creating and attaching a jejunal pouch.

Duodenal Atresia and Stenosis
Clinical Findings
Atresia and stenosis are related congenital obstructive anomalies of the proximal duodenum attributable to failed canalization of the duodenum during the 8th to 10th weeks in utero. Atresia is characterized by complete luminal occlusion; stenosis is characterized by incomplete occlusion. Stenosis can take several forms, including segmental narrowing and a diaphragm or web with one or more openings partially occluding the duodenal lumen.
Duodenal atresia has an incidence of 1 in 10,000 live births and is much more common than stenosis. The newborn usually has bilious vomiting because the atresia is located distal to the ampulla of Vater in 75% of patients. Duodenal stenosis or web, on the other hand, may not become clinically evident until later in life. The small lumen may become plugged with food only after the infant graduates from a liquid to a solid diet.
Duodenal atresia and stenosis are associated with other anomalies, including trisomy 21, which is found in approximately one third of infants who have atresia or stenosis. Other associations include the VACTERL spectrum of abnormalities, cardiac anomalies, and malrotation.
Annular pancreas may be found in up to 20% of patients with duodenal atresia or stenosis but is rarely the primary cause of the duodenal obstruction. Because the surgical approach to duodenal stenosis does not differ significantly when annular pancreas is present, an extensive preoperative evaluation is unnecessary.
Imaging Findings
Duodenal atresia and other congenital obstruction of the duodenum are reliably diagnosed with prenatal ultrasonography at or before 20 weeks of gestation. Typically, the sonogram shows a double bubble sign of fluid-filled, dilated fetal stomach and duodenum, although this observation has also been reported as a transient finding in otherwise normal fetuses ( Fig. 116-3 ).

After birth, the abdominal plain radiograph is usually diagnostic and shows the double bubble, with gas filling a distended stomach and proximal duodenum with no distal gas ( Fig. 116-4 ). The radiograph may be nondiagnostic if the stomach and duodenum are decompressed by vomiting or a nasogastric tube. If plain radiographs are not diagnostic, air or barium can be injected through the nasogastric tube. In incomplete duodenal obstructions, a double bubble is accompanied by gas in distal bowel. An important condition that may mimic duodenal atresia or stenosis on plain radiographs is malrotation with midgut volvulus.

Infants with clinical and plain radiographic evidence of complete or partial high-grade duodenal stenosis will rarely require additional imaging with contrast upper gastrointestinal examination because surgical intervention is required to relieve the obstruction. Surgery can be delayed if necessary for duodenal atresia or stenosis but not for malrotation and midgut volvulus. If a delay in surgery is contemplated, upper gastrointestinal examination or ultrasound should be performed to confirm absence of malrotation.
Complete obstruction of the duodenum with failure of contrast material to pass beyond the atretic segment and duodenal bulb dilation out of proportion to the stomach are typical findings on upper gastrointestinal examination in atresia. In duodenal stenosis, narrowing of the second portion of the duodenum is seen, but the corkscrew appearance of the duodenum and displacement of the duodenojejunal junction (DJJ) seen in malrotation and volvulus are absent. Duodenal diaphragm or web, which may also be called intraluminal diverticulum, appears as a fine linear filling defect within the barium-filled duodenum ( Fig. 116-5 ). In older children, the diaphragm that has been stretched for years may take on the appearance of a windsock on both barium and ultrasonographic studies.

Duplication Cyst
Clinical Findings
Duplication cysts are rare in the stomach and duodenum. Duplications that arise from the gastroesophageal junction may contain respiratory and enteric tissue, and they probably represent a bronchopulmonary foregut malformation. If a cystic mass is discovered at the gastroesophageal junction, chest radiographs should be scrutinized for a mediastinal or pulmonary component to the cyst. Duplication cysts of the distal stomach and duodenum may be enteric or neurenteric in origin. In early intrauterine life, a neurenteric canal connects ectoderm to endoderm and passes through dorsal neural folds. Persistence of this or an accessory canal (i.e., split notochord syndrome) gives rise to a series of anomalies, including diastematomyelia, hemivertebrae, and enteric cysts. Most gastric and duodenal cysts are considered the result of abnormal canalization of the intestinal tract. Duplication cysts are often diagnosed prenatally.
The common features of gastric or duodenal duplication cysts include obstruction, palpable upper abdominal mass, gastrointestinal bleeding, and respiratory distress. Most duplication cysts are diagnosed during the first year of life.
Imaging Findings
Gastric duplication cysts can be evaluated by upper gastrointestinal examination, ultrasonography, or both ( Fig. 116-6 ). At fluoroscopy, a “beak” results when contrast material in the gastrointestinal tract lumen surrounds the proximal portion of an obstructive duplication cyst. On ultrasound, enteric duplication cysts can usually be recognized by a characteristic pattern of echoes in the bowel wall, which mirrors that seen in the native bowel. This pattern has been termed the gut signature and consists of an inner hyperechoic ring representing mucosa and submucosa and an outer hypoechoic ring representing muscularis propria.

Malrotation
Clinical Findings
The developing gastrointestinal tract normally has two zones, the duodenojejunal and the cecocolic, that seem to be able to pull adjacent bowel along with them. Before the 8th week of intrauterine life, the duodenojejunal segment returns to the peritoneal cavity from the omphalus, having coursed counterclockwise to the left of the midline, under the superior mesenteric artery (SMA). Later, after the 10th week of life, the cecocolic segment undergoes a counterclockwise rotation toward the right lower quadrant. Intestinal malrotation refers to improper completion of the normal rotational process, resulting in formation of abnormal mesenteric attachments (i.e., Ladd’s bands) and shortening of the mesenteric base. Arrest of rotation may occur at any phase of development or involve only a part of the midgut, resulting in a wide spectrum of rotational anomalies ranging from nonrotation (in which the small bowel lies to the right of the mesenteric vessels and the colon to the left) to minor degrees of cecal elevation.
Particular attention should be paid to two critical anatomic landmarks in malrotation. First is the ligament of Treitz, which is a suspensory ligament of connective tissue and smooth muscle that runs from the root of the SMA to the junction of the fourth portion of the duodenum with the jejunum. Its presence is inferred on an upper gastrointestinal examination by normal position of the duodenal-jejunal junction (DJJ) in relation to the stomach, proximal duodenum, and spine. The DJJ and ligament of Treitz are located in the left upper quadrant of the abdomen in normal individuals but are displaced medially and inferiorly in those with malrotation. Second is the third and fourth portions of the duodenum, which are fixed in the retroperitoneum under the SMA in normal individuals but freely movable in the peritoneal cavity in those with malrotation.
It is not the malrotation that causes symptoms but rather complications arising from the malrotated, abnormally positioned intestine. The most grave is a midgut volvulus that results from twisting of the mesentery around the abnormally narrowed and shortened vascular pedicle. Volvulus may be fixed or intermittent and is a life-threatening emergency because of potential for bowel ischemia and necrosis. Obstruction may also result from Ladd’s bands across the duodenum. Affected individuals are usually symptomatic in the first year of life, but malrotation can be manifested at any age. Bilious emesis is the classic symptom, and a high index of suspicion for malrotation and volvulus should be maintained for those infants presenting in this manner. On occasion, a child may tolerate obstruction from intermittent volvulus and come to medical attention because of episodic pain or symptoms of malabsorption. Chronic, intermittent volvulus is a cause of secondary lymphangiectasia and chylous ascites.
Urgent surgical treatment of malrotation is indicated to avoid the potentially catastrophic complications of bowel necrosis associated with volvulus. The definitive treatment is Ladd’s procedure, in which the midgut volvulus is untwisted, Ladd’s bands are divided, cecum is mobilized, appendix is removed, and mesentery is widened, with placement of small bowel in the right hemiabdomen and colon in the left hemiabdomen.
Imaging Findings
Plain radiographs of patients with malrotation or volvulus show a variety of appearances ranging from paucity of distal bowel gas to unusual position of the air-filled stomach or intestinal loops, gaseous dilation of the stomach or duodenal bulb due to obstruction, and grossly distended air-filled loops with mural thickening if there is a closed-loop distal bowel obstruction and ischemia from volvulus. Most often, however, the findings on plain radiography are normal.
There is considerable debate in the literature concerning the best diagnostic approach to document position of bowel and presence of obstruction in suspected midgut volvulus. The methods described in the following paragraphs are not foolproof, and ultimately, because the stakes of missing a malrotation are so high, in inconclusive cases, the radiologist should not hesitate to repeat a study or to use a different, complementary modality to reach the correct diagnosis.
A carefully controlled upper gastrointestinal series, with delivery of barium or water-soluble contrast material orally or through a nasogastric tube placed in the stomach or, better yet, in the proximal duodenum, is generally considered the “gold standard.” The critical anatomy to document is the DJJ in a well-positioned straight frontal view of the first passage of contrast material through the duodenum. The DJJ, and by inference the ligament of Treitz, is considered normal when it is at or above the level of the superior end plate of the L2 vertebral body or the duodenal bulb and to the left of the left pedicle of this vertebral body. On lateral views, the second and third portions of the duodenum are posterior because they are retroperitoneal.
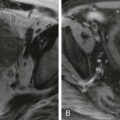
Displacement of the DJJ inferiorly or medially on the anteroposterior view and anteriorly on the lateral view is a sign of malrotation ( Fig. 116-7 ). Ladd’s bands typically cause a complete or partial obstruction of the duodenum; midgut volvulus causes a corkscrew, Z, or ribbon-like appearance of the duodenum and jejunum, sometimes with proximal dilation and partial obstruction of the duodenum ( Fig. 116-8 ).


On occasion, normal anatomic variations of the duodenum may be difficult to distinguish from genuine malrotation, or the DJJ may not be visualized because of technical factors. Normal variations that may mimic malrotation include inferior displacement of the DJJ by dilated stomach or bowel in infants with gastric overdistention or distal bowel obstruction; mobility of the DJJ in children younger than 4 years; and redundancy of the second portion of the duodenum, also known as mobile duodenum or water-trap duodenum. In cases in which the position of the DJJ is equivocal, the position of the cecum can be evaluated by either following the contrast material into the colon or performing a contrast enema. However, the cecum, although usually located in the right lower quadrant, may have a wide range of normal in infants and may be in a normal position even with malrotation. Of children with surgically proved malrotation, only 87% had an abnormally positioned cecum compared with 97% with an abnormally positioned DJJ.
Several sonographic features of malrotation have been described. Inversion of locations of the SMA and superior mesenteric vein (SMV) is one finding that has been described in malrotation. The SMV usually lies to the right of the SMA on a transverse image at the level of the junction with the portal vein. If the SMV is to left of the SMA, the diagnosis of malrotation should be entertained. However, this is an inconstant finding, with sensitivity of 67% to 100% and specificity of 75% to 83%, and normal relationships are present in malrotation and inverted relationships are present without malrotation. The whirlpool sign, indicating a midgut volvulus, can be seen on ultrasound when the bowel, SMV, and SMA are twisted and wrapped around the vascular mesentery ( Fig. 116-9 ). Finally, sonographic demonstration of the third portion of the duodenum in a retroperitoneal position between the SMA and the aorta is a reliable but not infallible sign of normal intestinal rotation. Likewise, absence of a retroperitoneal duodenum is a strong indicator of malrotation.

After Ladd’s procedure for malrotation and volvulus, upper gastrointestinal examination shows the expected postoperative appearance of persistently displaced DJJ, small bowel in the right hemiabdomen, and colon in the left hemiabdomen ( Fig. 116-10 ). Other possible complications include adhesions and small bowel obstruction, reported in up to 24% of patients, and recurrent volvulus, reported in up to 7% of patients.

Acquired Diseases
Gastric Perforation
The incidence of acute spontaneous gastric perforation in neonates is declining. Predisposing factors include acute distention of the stomach, ischemic necrosis associated with perinatal asphyxia, and distal obstruction such as annular pancreas or duodenal stenosis. Plain radiographs show pneumoperitoneum and absence of a gastric air-fluid level on upright views. Neonatal duodenal perforation is rare.
Gastric perforation in older children occurs in the following clinical settings: perforated peptic ulcer, dermatomyositis (although duodenal perforation is more common), migration of tubes and catheters through the gastric wall, and prior ingestion of caustic substances. Complications of the Nissen and other fundoplications include gastric bloat and gastric rupture or infarction when patients also have distal small bowel obstruction. Blunt trauma to the upper abdomen, when the stomach is distended, occasionally results in gastric rupture.
Pyloric Stenosis
Clinical Findings
Hypertrophic pyloric stenosis, which occurs in approximately 3 of 1000 infants, is one of the most common indications for surgery in infants. This disorder with familial predisposition is of unknown etiology but probably results from a complex interaction of genetic and environmental factors. Males, especially first-born males, are affected more frequently.
Classically, pyloric stenosis is manifested in a previously healthy infant between the age of 2 and 12 weeks with repeated nonbilious emesis that is sometimes forceful or projectile. The gradual onset of the symptoms may be mistaken for new or worsening gastroesophageal reflux. Presentation outside of this age range or emesis that is bilious should prompt evaluation for alternative diagnoses, such as malrotation. Pyloromyotomy, which can be performed nonemergently, is the treatment of choice for pyloric stenosis. Because of better cosmetic result, shorter hospital stays, and lower wound infection rates, laparoscopic surgery is increasingly favored over an open procedure.
Imaging Findings
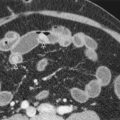
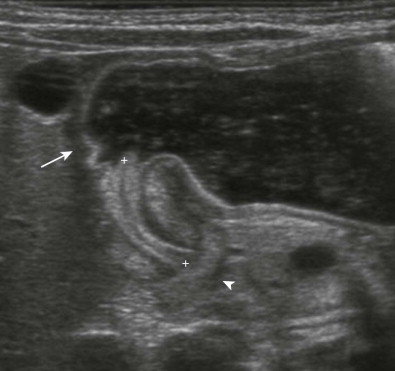
Ultrasonography is the established imaging examination for the diagnosis of pyloric stenosis and has been found to be highly sensitive and specific. In contemporary medical practice, the reliance on imaging has progressed to the point that ultrasound is now considered an intrinsic part of evaluation for pyloric stenosis, whereas the classically described palpable “olive” is found on physical examination with decreasing frequency. Features easily depicted by sonography include a thickened, hypoechoic pyloric muscle (doughnut or cervix sign) and a double layer of redundant echogenic mucosa (sonographic double-track sign) ( Fig. 116-11 ). A muscle thickness of more than 3.0 to 3.5 mm measured in the long axis of the pylorus is a reliable indicator of pyloric stenosis, regardless of the patient’s age or weight. A pyloric channel length of more than 15 to 18 mm is also considered abnormal. As important as the quantitative measurements in diagnosis of pyloric stenosis is the morphologic appearance of the pylorus and the real-time observation of little or no passage of gastric contents through the pylorus.
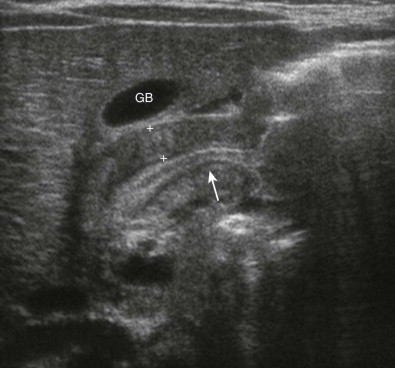
In pyloric stenosis, the thickened muscle is a fixed abnormality that does not change with time. Pylorospasm, which is a condition in which the muscle enlargement is transient rather than fixed and which is treated nonoperatively, may mimic pyloric stenosis on sonography. The muscle may be thickened, but the degree of thickening is often less pronounced than in pyloric stenosis, usually less than 3.0 mm. In addition, whereas muscle thickening is persistent in pyloric stenosis, it is intermittent in pylorospasm, with occasional relaxation of the pyloric muscle allowing passage of gastric contents. Thus, extending the length of time of observation of the pylorus to at least 5 to 10 minutes is an essential part of the sonographic study that can prevent false-positive diagnosis of pyloric stenosis. In equivocal cases, repeated sonography in 1 to 3 days can be performed to detect early or evolving pyloric stenosis.
Sonography should be performed with a high-frequency linear array transducer placed with the liver as an acoustic window. To allow adequate filling of the antrum with fluid and assessment of patency of the pyloric channel, the infant can be turned to the right posterior oblique position and fluid can be given orally ( Fig. 116-12 ). These maneuvers can help demarcate the landmarks denoting the beginning and end of the pyloric channel, namely, the prepyloric antrum and the duodenal bulb. Fluid should not be given if the stomach is already distended as an overdistended stomach displaces the pylorus behind the stomach, making visualization difficult and possibly leading to false-negative results.
Contrast upper gastrointestinal examination is rarely performed if pyloric stenosis is the primary diagnostic consideration, but radiologists should still recognize findings because unexpected pyloric stenosis is occasionally found in infants undergoing upper gastrointestinal examination for suspected reflux. Upper gastrointestinal examination is also an alternative examination in the event that sonographic expertise is not available. The classic features of pyloric stenosis on barium radiography include partial or complete gastric outlet obstruction, hyperperistalsis of the stomach, elongation of the pyloric channel, single (i.e., string sign) or double (i.e., train track sign) streaks of barium within the compressed lumen of the channel, and the shoulder sign of a pyloric mass indenting the barium-filled stomach and the base of the duodenal bulb ( Fig. 116-13 ).
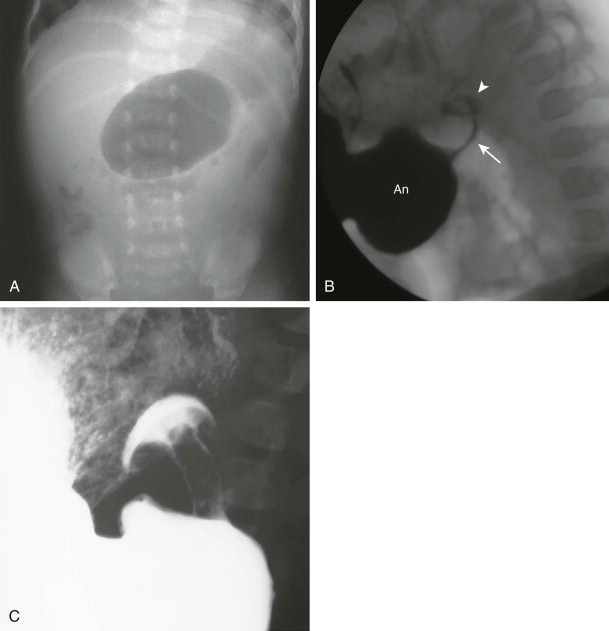
The radiographic appearance in the immediate postoperative period after pyloromyotomy is difficult to interpret because it is similar to that of preoperative studies, with a string sign and antropylorospasm ( Fig. 116-14 ). By 6 weeks, however, the sonographic and radiographic appearance should be normal for most patients. Until then, there is often asymmetry of the channel as the mucosa eventrates through the defect of the “cracked” muscle. Incomplete pyloromyotomy results in persistence of an elongated, narrowed channel with poor gastric emptying. Patients who have had a past history of successful pyloromyotomy may be left with some antropyloric dysfunction, which has led to retention of foreign bodies (e.g., coins) in some patients.
Gastric and Duodenal Hematomas
Clinical Findings
Gastric hematoma from blunt abdominal trauma is unusual. Duodenal hematoma is more common because the duodenum is sandwiched between the spine and the anterior abdominal wall of the epigastrium. A typical history in such cases is a child falling onto the handlebars of a bicycle or being struck in the abdomen during play or an athletic event. Duodenal hematomas are associated with pancreatic injury because the pancreas is also located in the retroperitoneum in a position vulnerable to blunt epigastric trauma.
Child abuse should be considered when any child has a suspicious history. Other risk factors for duodenal hematoma include Henoch-Schönlein purpura, bleeding associated with leukemia, coagulopathies, idiopathic thrombocytopenia purpura, endoscopic biopsy, and anticoagulant therapy. Surgery is mandatory when perforation is present, but otherwise most uncomplicated duodenal hematomas are managed conservatively.
Imaging Findings
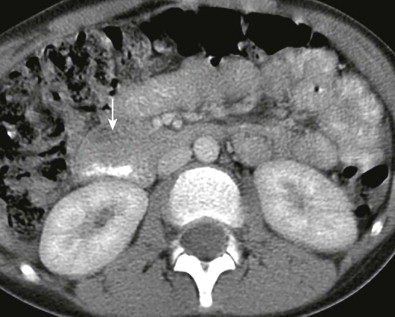
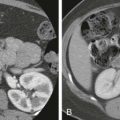
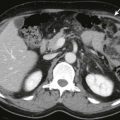
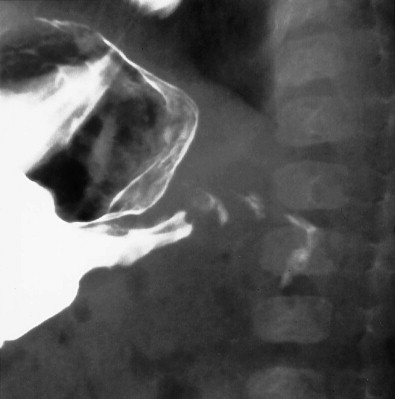
The diagnosis of duodenal hematoma can be suggested by plain radiographs that demonstrate gastric distention, soft tissue mass in the right hemiabdomen, and sparse distal gas. Retroperitoneal air can be seen on the plain radiograph or computed tomography (CT) scan as a sign of transmural leakage. Upper gastrointestinal study with contrast material shows the intramural mass effect of the hematoma but may not demonstrate the perforation if the hematoma is plugging the mural rent ( Fig. 116-15A ).
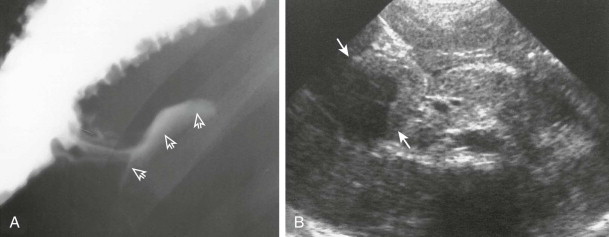
Ultrasonography can demonstrate and monitor the hematoma and adjacent pancreatic injury that is commonly present, but it cannot reliably demonstrate perforation ( Fig. 116-15B ). CT is the preferred method of imaging if there has been severe upper abdominal trauma, especially crush injury, because it can image all organs well ( Fig. 116-16 ). However, CT can miss subtle cases of duodenal rupture. As the hematoma resolves, perforation and duodenal diastasis may become apparent, and every child with a duodenal hematoma must be carefully watched during the first 7 to 10 days after trauma.