- •
Assess for the morphology of the larger ventricle (left ventricle).
- •
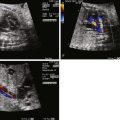
Identify the atrioventricular valve connections to the left ventricle, and assess size and degree of insufficiency, if present.
- •
Identify the atrial septum and direction of shunting across.
- •
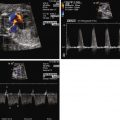
If the left atrioventricular valve is small, assess left-to-right gradient across the atrial septum and interrogate the pulmonary veins for Doppler flow pattern in order to determine whether there is obstruction to left atrial egress.
- •
Identify the position of the small right ventricle as either anterior and rightward, anterior and leftward, or superior.
- •
Locate and identify the size of the ventricular septal defect communication between the large left ventricle and the smaller right ventricle.
- •
Identify the great arterial position and origins as either from large left or from small right ventricle.
- •
Determine the nature of the pathway patency from the large left ventricle to the pulmonary artery and to the aorta.
- •
Assess direction of flow in the ductus arteriosus, antegrade from pulmonary artery to aorta or retrograde from aorta to pulmonary artery.
- •
Assess the size of the ascending aorta and aortic arch when the aorta arises from a small hypoplastic right ventricle and the ventricular septal defect is restrictive.
- •
Identify the direction of flow in the transverse aorta when there is aortic outflow obstruction.
- •
Assess the size of the branch pulmonary arteries when there is pulmonic outflow obstruction.
- •
Look for irregularity in heart rhythm, which may reflect heart block.
Anatomy and Anatomical Associations
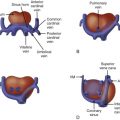
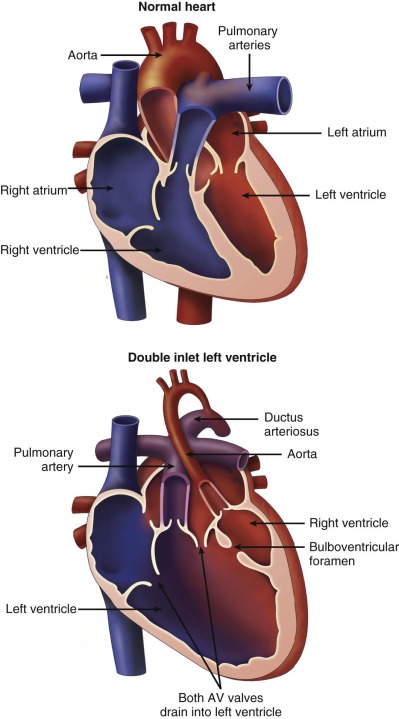
Double-inlet left ventricle (DILV) is a term used to describe the cardiac malformation in which there are two atrioventricular (AV) valves that drain into a single, large dominant ventricle of left ventricular morphology, which is associated with a diminutive opposing rudimentary outflow chamber. Controversy surrounds the nomenclature of this malformation, and other terms used to describe this anomaly have included single ventricle and a univentricular heart of the left ventricular type. The main ventricular chamber is of left ventricular morphology with characteristic fine trabeculations. The rudimentary outflow chamber of right ventricle (RV) origin consists only of trabecular and outlet portions and is often in communication with the single left ventricle (LV) through a ventricular septal defect (VSD). The VSD is also often referred to as a bulboventricular, or outlet, foramen. In this anomaly, the interventricular septum is displaced and malformed, but not absent. The rudimentary right ventricular chamber is located anterior to the main ventricle, either rightward ( d -loop) or leftward ( l -loop), and is separated by an anterior trabecular septum. Both AV valves are posterior to the trabecular septum and there is no intervening inlet septum between the two.
Diagnosis of DILV excludes those patients with an unbalanced common AV valve. Both AV valves in DILV are in fibrous continuity with the posterior great artery. The RV is variable in size ranging from slitlike to near 80% of the size of a normal RV. There may be either concordance (normal) or discordance (transposition) of the ventriculoarterial relations.
DILV is one of the most common forms of single ventricle and is regarded as the “classic form” because both AV valves communicate into a common chamber. Van Praagh and coworkers distinguished three primary subtypes of DILV based on the relationship of the great arteries: (1) type I DILV has normally related great arteries, (2) type II has a rightward and anterior aorta and rightward outlet chamber, and (3) type III has a leftward anterior aorta. DILV with a hypoplastic subpulmonary, rightward RV and normally related great arteries (type I) is classically referred to as the “Holmes heart” and is relatively rare in our current experience. Type II was observed in 21% of cases of DILV in Van Praagh and coworkers’ series. In this form, the outlet chamber is anterior and rightward, consistent with d -looped ventricles, and there is d -transposition of the great arteries, segments {S,D,D}. This type is associated with obstruction of the bulboventricular foramen and, therefore, subaortic stenosis, because the aorta arises from the small chamber. Arch anomalies are reported in approximately 50% of cases.
Type III is the most common form of DILV, accounting for 54% of the cases reviewed by Van Praagh and coworkers. Type III consists of DILV with a left-sided, subaortic, hypoplastic right ventricle ( l -loop ventricles) and l -transposition of the great arteries, segments {S,L,L}. Subaortic stenosis is present in approximately 67% of patients with this morphology due to a small bulboventricular foramen or obstruction by left AV valve tissue ( Figure 29-1 ).
Other arterial relations with DILV have included double-outlet arterial connections, with both great arteries arising from the rudimentary right ventricular chamber. Single-outlet connection, most frequently pulmonary atresia, and rare cases of truncus arteriosus have been reported. Bevilacqua and colleagues described a similar spectrum of variety in which out of 57 patients with DILV, 14% had d -looped ventricles with normally related great arteries, 23% had d -looped ventricles with d -transposition of the great arteries, and 63% had l -looped ventricles with l -transposition of the great arteries.
Varying degrees of straddling of the AV valves have been noted in patients with DILV. When straddling occurs, the AV valve that straddles is almost always on the same side as the rudimentary right ventricular chamber. Atresia, stenosis, hypoplasia, and thickening of either AV valve may occur. A parachute-like deformity of the AV valves has also been reported. Shiraishi and associates found a stenotic AV valve in 30% of patients with double-inlet ventricle, most commonly occurring in the left (pulmonary venous) AV valve. When one AV valve is atretic, DILV can be diagnosed if the atretic valve plate overrides the ventricular septum and is committed more than 50% to the LV. Abnormal valves may also lead to AV valve regurgitation.
Pulmonary outflow tract obstruction in DILV occurs with either concordant or discordant ventriculoarterial relations (pulmonary artery from RV or pulmonary artery from LV, respectively). When subpulmonary obstruction occurs within the left ventricular chamber in discordant ventriculoarterial relationship type DILV, it is most frequently due to posterior deviation of the infundibular septum, anomalous attachments of the right AV valve, or herniation of valvular tissue into the pulmonary outflow tracts. When there is concordant ventriculoarterial relation and the pulmonary artery arises from the small RV, subpulmonary obstruction has been reported in 50% of patients with a Holmes heart and can result in hypoxia at birth. Severe pulmonary stenosis and annular hypoplasia can also occur. The pulmonary valve can be thickened and is often bicuspid.
Subaortic obstruction occurs with ventriculoarterial discordance and is located primarily at the level of the interventricular communication. Restriction at this level primarily occurs with a muscular type defect. An interventricular communication, which is smaller in dimension than the aortic root, can be at risk for obstruction over time. Restriction can be severe and may lead to ductal-dependent circulation. Such patients often have coexisting anomalies of the aortic arch including coarctation or interruption. Subaortic obstruction can also be acquired secondary to severe ventricular hypertrophy from muscle bundles within the hypoplastic right ventricular chamber. This occurs more often in patients with associated right AV valve atresia and in patients with DILV and transposed great arteries who are status postpulmonary artery banding. Pulmonary artery banding in such patients has been associated with progressive ventricular hypertrophy and restriction of the interventricular communication.
Conduction abnormalities have also been associated with DILV due to the abnormal course of the conduction tissue. These abnormalities are found most frequently in patients with discordant arterioventricular relations ( l -looped ventricles). Patients with this anatomy are at risk for development of complete heart block.
Frequency, Genetics, and Development
Single-ventricle anatomy accounts for 1.33% to 2.4% of congenital heart disease with a prevalence of 0.054 to 0.08 per 1000 live births. Approximately 60% to 70% of all univentricular hearts are characterized by dominant left ventricular morphology.
The developmental origins of DILV are not fully understood. It is speculated that arrest or incomplete movement of the AV septum or the conotruncus results in the malalignment of the inflow and outflow tracts. When the AV septum fails to shift to the right, the right and left AV canals communicate with the LV, forming a DILV. In addition, an abnormality of cardiac looping is often associated with DILV. In a Holmes heart, the interventricular communication represents the primitive bulboventricular foramen.
There are no known specific extracardiac findings or genetic syndromes common to patients with DILV. There is currently no known genetic etiology for DILV.
Prenatal Physiology
The impact of this anomaly on fetal physiology depends primarily on the presence, type, and degree of outflow tract obstruction. Owing to the presence of the ductus arteriosus in fetal circulation and the fact that maternal oxygenation is provided by placental flow, there is minimal hemodynamic compromise to the fetus. If severe aortic outflow tract or aortic arch obstruction is present, as is possible in the case of a rudimentary chamber with ventriculoarterial discordance, systemic perfusion is provided by the right-to-left flow through the ductus arteriosus with retrograde perfusion to the cerebral circulation. When pulmonary outflow is severely obstructed, there is reversal of ductal flow with left-to-right (aorta–to–pulmonary artery) perfusion of the pulmonary vasculature. DILV is not usually associated with significant AV valve regurgitation, ventricular dysfunction, or tachyarrhythmias and, therefore, is not typically associated with hydrops.
Prenatal Management
Prenatal management of this anomaly focuses primarily on accuracy of the diagnosis in order to provide counseling for the family regarding the various options for management. A significant challenge on echocardiography is to distinguish which great vessel is arising from the small outflow chamber—the pulmonary artery or the aorta. In conditions of a very small aorta, the head vessels may falsely appear as if they arise form the large ductal arch, confusing it for the aorta. Identifying which great vessel is small in DILV has ramifications for counseling. If the aorta is small, a modified Norwood operation may be necessary, which carries relatively high risk. If the pulmonary artery is small, a lower-risk initial operation, a Blalock-Taussig shunt, is undertaken.
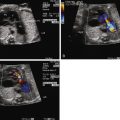
Fetal echocardiography in DILV should focus on the outflow tract and great vessels relationship as well as delineation of all systemic and pulmonary venous connections, atrial and ventricular anatomy, and structure and function of the AV valves. The fetus is to be followed with serial fetal echocardiography on a regular basis, primarily to monitor for progression of outflow tract obstruction and AV valve regurgitation. Our practice is to see such patients every 4 weeks until term.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree