- •
Origin of the great vessels from the right ventricle.
- •
Relationship of the great vessels to each other (aorta is typically to the right of the pulmonary artery).
- •
Identify the location of the ventricular septal defect in relation to the great vessels as either subpulmonic or subaortic.
- •
Identify the presence or absence of pulmonic or aortic outflow obstruction.
- •
If there is pulmonic outflow obstruction, identify the direction of shunting across the ductus arteriosus.
- •
If there is aortic outflow obstruction, assess the aortic arch for possible coarctation of the aorta or interruption of the aortic arch.
Anatomy and Anatomical Associations
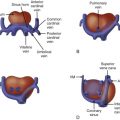
Double-outlet right ventricle (DORV) is a type of congenital heart disease in which both great arteries arise from the right ventricle (RV). More specifically, it is a ventriculoarterial alignment that occurs when both the aorta and the pulmonary artery are positioned at least 50% over the RV, and there is a lack of aortic–mitral annular fibrous continuity.
DORV is not a single, specific abnormality but, rather, defines an anatomical relationship that encompasses a wide spectrum of different congenital heart defects with varying physiologies. A ventricular septal defect (VSD) almost always accompanies DORV and is the only path out of the left ventricle (LV). DORV can be classified into different subtypes based on the location of the VSD: (1) DORV with subaortic VSD, (2) DORV with subpulmonic VSD, and (3) DORV with a double, or uncommitted, VSD. The first two types are the most common and are discussed here. DORV with an atrioventricular canal type VSD can be a type of DORV with uncommitted VSD and typically occurs in heterotaxy syndrome; this is addressed in another chapter. DORV with mitral hypoplasia and associated left ventricular hypoplasia can also occur; this is discussed separately in Chapter 22 .
Variations in the relationship of the VSD to the great vessels are determined based on the three-dimensional spatial relationship between the plane of the ventricular septum and the position of the great vessels to the septum and to each other. The relative position of the vessels is in turn dependent upon the presence and amount of conus muscle underneath. Whereas, in the normal heart, there is no subaortic conus and only subpulmonary conus, in DORV, there can be conus beneath one or both great vessels to varying degrees, lifting and positioning the great vessels over the ventricular septum and VSD, creating the different great vessel–to–VSD relationships that exist. Furthermore, the position of the conal septum and its deviation and malalignment away from the plane of the muscular septum contribute to the development of sub–great vessel narrowing (subpulmonary narrowing in the subaortic VSD type DORV; subaortic narrowing in the subpulmonic VSD type of DORV).
The VSD–to–great vessel relationship determines the nature of the defect that is seen. VSD position determines where the outflow of the LV and blood exiting the LV will be directed ( Figure 16-1 ). The VSD is classified as subaortic when it is more closely related to the aortic valve. In such cases, left ventricular outflow is directed into the aorta and pulmonary stenosis is common. The physiology and clinical manifestations are similar to that of a simple VSD, if there is no pulmonic stenosis, or similar to tetralogy of Fallot (TOF), if there is pulmonic stenosis. Common associated anomalies in DORV with subaortic VSD include pulmonary stenosis, atrial septal defects, mitral valve abnormalities, and left superior vena cava to coronary sinus.
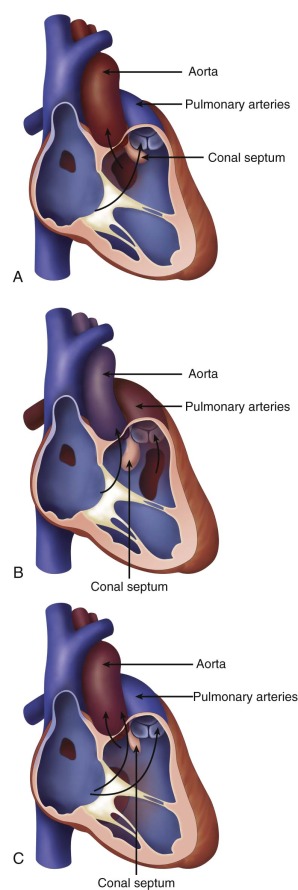
The VSD is classified as subpulmonic when it is more closely associated with the pulmonary valve. In such cases, left ventricular outflow is directed into the pulmonary artery. The physiology and clinical manifestations are similar to that of transposition of the great arteries (TGA) because oxygenated blood from the LV is ejected into the pulmonary circulation. Common associated anomalies in DORV with subpulmonic VSD include aortic arch hypoplasia, coarctation of the aorta, and left superior vena cava to coronary sinus. The conal (infundibular) septum may be malaligned beneath the aorta causing variable degrees of subaortic narrowing. In a particular form of DORV with subpulmonic VSD, called the “Taussig-Bing” anomaly, there is bilateral conus underneath both great vessels, the aorta and pulmonary artery are side by side, and there is arch hypoplasia, or coarctation of the aorta.
In DORV in general, the aorta is almost always more rightward than normal and the relative arrangement is for the great vessels to lay more side by side than usual. As such, coronary artery anomalies with aberrant insertion may occur. Furthermore, a rightward-positioned aorta is associated with the rare anomaly of leftward “juxtaposition of the atrial appendages.” In this condition, the right atrium is its normal position; however, the right atrial appendage is positioned over on the left next to the left atrial appendage.
Frequency, Genetics, and Development
The incidence of DORV is approximately 0.2 per 1000 live births with the subaortic defect being the most common form, occurring approximately 50% of the time. To date, no specific genetic abnormality is associated with DORV. The developmental abnormalities that lead to DORV are likely related to a failure of the normal rotation of the conus with persistence of both great vessels from the RV.
Prenatal Physiology
The hemodynamic alterations in the fetus with DORV are usually mild and well tolerated. Oxygenated blood from the placenta returns to the left heart across the foramen ovale and will course across the VSD to the ascending aorta if there is a subaortic VSD and into the pulmonary artery if there is a subpulmonic VSD.
In the subaortic VSD type, when mild pulmonary stenosis is present, antegrade flow is ejected by the RV into the pulmonary circulation and antegrade flow through the ductus arteriosus to the descending aorta. If the pulmonary stenosis is severe, blood supply to the developing lungs is derived from the aorta via retrograde flow across the ductus arteriosus. Severe pulmonic stenosis will also divert blood away from the pulmonary artery and the ascending aorta will carry more of the combined cardiac output, but at slightly lower oxygen saturation.
In the subpulmonic VSD type, the physiology is similar to that of TGA. The combination of (1) right-to-left shunted atrial-derived blood and (2) small amount of pulmonary venous return that traverses the mitral valve is ejected out of the LV, through the VSD, into the pulmonary circulation with the predominance of it directed to the ductus arteriosus and descending aorta. If there is marked conal septal deviation beneath the aortic valve, there may be limitation of blood flow into the aorta, which may explain why many such fetuses exhibit arch hypoplasia and coarctation of the aorta.
Prenatal Management
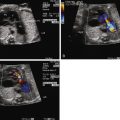
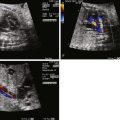
Imaging of DORV involves careful evaluation of the position of the great vessels in relation to the ventricles and the ventricular septum. The great vessels will arise from the heart parallel to each other and will not cross in space. In the four-chamber view, angling anteriorly, one will see a VSD; further anterior and superior angling will reveal the origin of the great vessels. On two-dimensional imaging, the relationship of the VSD to the great vessels can be determined. In subaortic VSD, the aorta is positioned rightward but adjacent to and just above the VSD. Another way to confirm presence of a subaortic VSD is in fact to identify absence of the ability to “connect” VSD flow with the pulmonary artery in any plane because the conal septum gets in the way of any such visual connection. In subpulmonic VSD, the pulmonary artery will commonly appear to straddle the ventricular septum. Sometimes, it is difficult to distinguish whether in fact the pulmonary artery is 50% over the RV or the LV, the former being DORV, the latter TGA with VSD.
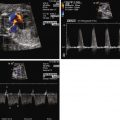
Color Doppler imaging will further indicate the direction of flow exiting the LV via the VSD. Oftentimes, a stream of color flow will be seen in a single plane exiting the VSD directed into either the aorta or the pulmonary artery. This is best determined in the four-chamber or long-axis views.
In cases of DORV with subaortic VSD with pulmonary stenosis, one must predict whether there will be ductal-dependent pulmonary blood flow and the need for prostaglandin (PGE) infusion in the immediate newborn period. Guidelines to those for TOF can be used. If the narrowest diameter of the pathway to the pulmonary circulation is less than half the diameter of the aorta, this should raise suspicion of the possible need for supplemental pulmonary blood flow through ductal perfusion and the need for PGE infusion at birth.
In cases of DORV and subpulmonic VSD, the pulmonary artery will be relatively large and analysis of the subaortic region and aortic arch are indicated. The degree of conal deviation beneath the aorta can be appreciated in the long-axis views. The aortic annulus should be assessed and measured, as should the ascending and transverse aorta, because the severity of hypoplasia of the aorta will dictate the postnatal surgical strategy.
If pulmonary stenosis or abnormalities of the aorta have been identified, it is recommended to serially evaluate the fetus to monitor for progression. DORV is considered a form of conotruncal anomaly; therefore, there is indication for analysis of the fetal chromosomes and an obstetrical ultrasound to evaluate for extracardiac anomalies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree