Fluid collections, lymphadenopathy, and cystic and solid masses are encountered routinely in the practice of gynecology and gynecologic oncology. For example, tuboovarian abscesses (TOAs) may be sequelae of pelvic inflammatory disease, diverticulitis, appendicitis, Crohn’s disease, or may be postoperative. Hematomas, lymphoceles, seromas, peritoneal inclusion cysts, and ovarian cystic lesions may be symptomatic and require treatment.
The ability to aspirate and drain fluid collections and biopsy solid masses are among the most important and widely used advances offered by interventional radiology. Percutaneous drainage is now standard therapy for abscesses in patients without another indication for surgery. Aspiration of symptomatic cystic lesions such as ovarian cysts or lymphoceles can be effective. Although surgery is the standard approach for cystic and solid lesions where there is a suspicion of malignancy, biopsy may be helpful when patients are poor surgical candidates, pregnant, have only one ovary and are premenopausal, or are being considered for neoadjuvant chemotherapy before surgical resection.
Percutaneous access is classically obtained via a transabdominal approach using ultrasound (US), computed tomography (CT), or, rarely, magnetic resonance imaging (MRI) guidance. When a transabdominal approach to pelvic lesions is considered, the bladder, bowel, vasculature, and pelvic bony ring can present obstacles to access. Other innovative routes to pelvic lesions have been used, which are critical to the armamentarium of the interventionalist; these include transgluteal, transvaginal, and transrectal approaches.
As important as assessing whether a fluid collection can be drained or whether a mass can be biopsied, it is just as imperative to consider whether it should. Because staging and treatment of the common gynecologic malignancies often require a multidisciplinary approach, intervention in suspected malignant lesions should be performed with appropriate oncologic consultation.
Description of Technical Requirements
Preprocedure Planning
The success of an aspiration, drainage, or biopsy is predicated on preprocedure planning. This includes review of the clinical history, relevant imaging, and laboratory workup.
Preprocedure planning begins with review of the clinical history and establishing the indication. Fluid collections encountered in patients with pelvic pain and fever may raise the suspicion of TOA. TOAs may just as often be due to bowel-related causes, that is, diverticulitis, appendicitis, or Crohn’s disease, as arise from pelvic inflammatory disease. TOA in postmenopausal patients may also be the presenting sign of a malignancy, and submission of aspirated or drained contents for cytologic analysis may be helpful. Postoperative collections may represent hematomas, lymphoceles, seromas, or urinomas. In addition to fluid characterization and treatment of infection, aspiration and drainage may be performed to alleviate symptoms of pain or pressure.
Review of the clinical history also helps identify steps to minimize the risk for procedure complications. History of easy bruising or bleeding diathesis should prompt review of coagulation or platelet parameters. Patients on anticoagulation or antiplatelet therapy are asked to withhold these medications before the procedure. The majority of procedures targeting deep pelvic lesions require patient sedation for both comfort and safety. Conscious sedation with intravenous administration of 1 to 4 mg of midazolam hydrochloride and 20 to 100 mcg of fentanyl citrate is usually sufficient. When sedating patients with severe cardiopulmonary disease, however, assistance of an anesthesiologist may be indicated. Preprocedure antibiotics are typically administered when fluid collections are aspirated or drained. Typically 1 g of ampicillin, 80 mg of gentamicin, and 600 mg of clindamycin are administered intravenously immediately before the procedure, and for those patients not on long-term intravenous antibiotic coverage, 150 mg clindamycin is administered orally every 8 hours for 5 days after the procedure.
Review of preprocedure imaging is important for several reasons. First, cross-sectional imaging will define the differential diagnosis for the target lesion and help tailor the procedure accordingly, for example, microbiology specimen for suspected infection, tissue core pathology specimen for suspected benign mass, flow cytometry specimen for suspected lymphoma, etc. Second, imaging will demonstrate the location of the mass relative to the surrounding structures and is crucial to planning a safe access route for the procedure. Third, preprocedure imaging enables the interventionalist to select the best modality to guide the procedure, US or CT.
Laboratory workup may also be important in identifying abnormalities that may raise the risk of the procedure, which may be modifiable. Abnormalities in coagulation profile or thrombocytopenia may need to be corrected ( Table 37-1 ). Infection may be suspected if there is leukocytosis; preprocedure antibiotics may be indicated in such circumstances. Renal function may be evaluated with calculation of an estimated glomerular filtration rate, particularly if intravenous contrast is to be administered during a CT-guided procedure.
| Parameter | Threshold | Action |
|---|---|---|
| PTT | >15 s | Stop heparin drip at least 2 hr before procedure |
| INR | >1.5 |
|
| Platelet count | <50,000/mcL |
|
Imaging Guidance
US offers real-time visualization, multiplanar capability, and avoids radiation. When drainage is performed, real-time visualization enables direct trocar of a catheter into a collection. US is generally the first-choice imaging modality for most aspiration, drainage, and biopsy procedures because of shorter procedure times and because the procedure can be performed at the bedside if necessary. Image quality can be variable, however, especially when a transabdominal approach is considered in obese patients. Additionally, bowel gas may obscure the deep field of the image, and collapsed bowel may be difficult to discern.
CT provides excellent tomographic visualization of anatomy. The needle trajectory, however, is generally restricted to the plane of imaging, usually axial. CT guidance is indicated in cases where the collection or lesion is not well demonstrated on sonography or if there is a concern for intervening structures such as bowel based on preprocedure imaging. CT guidance may also be helpful in obese patients. If available, CT fluoroscopy may be performed. Images may be reconstructed at a rate of up to 8 images/s and may be used in either continuous fluoroscopy mode or as a quick-check technique. Although the former takes full advantage of the ability to visualize interventions in real time, it is associated with higher radiation to the patient and operator. The quick-check technique involves the use of single CT fluoroscopic spot images to intermittently check needle position, reducing procedure duration compared with conventional CT guidance, and potentially reducing radiation compared with continuous fluoroscopy.
MRI combines advantages of US and CT—it provides multiplanar capability, avoids radiation, and offers excellent soft tissue contrast. It is associated with long procedure duration, however, and has limited availability. Open-bore systems offer improved operator access to the patient, but because these systems typically operate in the 0.2- to 0.5-Tesla range, the quality of imaging is poorer and the scanning times longer compared with closed-bore systems that operate in the 1- to 1.5-Tesla range. Closed-bore systems may restrict patient access, and as such, instrument manipulations are often performed with the patient outside the magnet. Closed-, short-, wide-bore 1.5-Tesla systems, however, may offer excellent image quality while allowing needle manipulations when the patient is in the magnet; this permits the use of MR fluoroscopy, which may be performed in open-bore designs. MRI guidance is more typically used in interventions involving hepatic dome and skeletal lesions where MRI may be the only modality with which the target is visualized. For gynecologic lesions, MRI guidance is almost never needed for drainage or biopsy when preprocedure diagnostic images are available to localize the target.
Techniques
Two general considerations relevant to gynecologic interventional techniques will be discussed. The first is choosing the path of access to the lesion; these include transabdominal, transgluteal, transvaginal, and transrectal routes. The second consideration is the description of the intervention itself, which includes aspiration, drainage, and biopsy.
Aspiration and Drainage of Fluid Collections
Indications
Aspiration refers to the act of removing fluid; if an indwelling catheter is to be left in, the procedure is referred to as a drainage . Aspiration or drainage may be performed to characterize fluid and may help to distinguish among collections such as abscess, hematoma, urinoma, or lymphocele. In general, purulent collections usually require indwelling catheter drainage, whereas noninfected collections may be either aspirated or drained. Aspiration of noninfected collections may be preferred to prevent risk for superinfection that can theoretically occur when a catheter is left in place.
The most common cause of pelvic abscess is recent surgery, including gynecologic and colorectal surgery. TOA secondary to pelvic inflammatory disease, appendicitis, or diverticulitis is another important cause. Whatever the cause of pelvic abscess, antibiotic therapy is required. The clinical effectiveness of drainage of pelvic abscesses is well documented, with therapeutic success exceeding 90%. Aspiration of TOAs may also be effective, although in some patients repeat aspiration is needed. Drainage is often preferred over aspiration to avoid the need for a repeat procedure or if all contents of a fluid collection cannot be removed by aspiration alone.
Contraindications
Contraindications to pelvic collection aspiration and drainage are rare. These include uncorrectable coagulopathy, cardiopulmonary instability, and lack of safe access route to the lesion. Aspiration may be preferred over drainage in sterile collections such as hematomas because prolonged catheter drainage may result in superinfection. Tumor abscesses may require lifelong catheter drainage.
Technique Description
Transabdominal Approach
The anterior transabdominal approach is the most commonly used route to intraabdominal collections. When possible, this route is preferred because it is generally easier technically and may be better tolerated by the patient.
Ultrasound Guidance
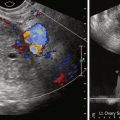
US guidance is the first-choice imaging modality because it is usually quicker, lacks radiation, and can be performed, if necessary, at the bedside. A preliminary transabdominal sonogram is performed; clear visualization of the collection is documented. If septae are seen within the collection, loculation should be suspected; drainage may be preferable and may require multiple catheters. Doppler sonography may be useful to evaluate for vessels in the planned needle or catheter tract. The most common technique is freehand—one hand controls the transducer, whereas the other controls the needle or catheter. However, a needle guide that attaches to the transducer is also available.
A decision may be made to perform aspiration first as a diagnostic procedure followed by drainage if necessary or, with suspected infected or multilocular collections, to place a drainage catheter immediately. After sterile preparation of the field, local anesthetic (e.g., 1% lidocaine) is applied along the planned needle or catheter tract from the skin to the surface of the collection. Aspiration may be performed with a needle (e.g., 20-gauge Chiba). If the collection is likely to require long-term drainage, a catheter (10 to 12 French) is preferred. If a needle is used, it is advanced under direct US visualization into the collection. The echogenic needle is aligned along the long axis of the transducer; visualization may be improved with a jiggling “in-and-out” motion. If a catheter is to be used for aspiration, the catheter is advanced with US guidance using a trocar delivery system containing a stiffening metal cannula and sharp inner stylet. Once in the collection, the inner stylet and cannula are removed.
A small 1- to 2-mL sample may be aspirated first and sent for microbiologic analysis; note is made of fluid color, viscosity, turbidity, and smell. If an adnexal collection is encountered or if the patient has a history of malignancy, samples may also be sent for cytology. Complete aspiration of the collection is subsequently performed. If sonography demonstrates a persistent collection, drainage should be considered.
The type of drainage catheter used is practitioner dependent. Generally locking pigtail-type catheters are more commonly used for abdominal and pelvic collections because the locking mechanism reduces the risk for catheter dislodgement in the setting of abdominal straining. These are available in 8- to 14-French sizes. Sump-type catheters are also available; these have a double-lumen design where the outer lumen prevents the sideholes from becoming blocked when abutting an abscess wall. These are typically available in 12- to 28-French sizes. In general, larger-bore catheters are useful for viscous collections such as hematomas. A 10- to 12-French catheter usually suffices for most abscesses. Once the catheter is placed using the direct trocar technique, fluid is completely aspirated, and the cavity is irrigated with normal saline. Finally a postprocedure image is obtained.
Computed Tomography Guidance
CT guidance is used when the target lesion or a clear path to it cannot be well demonstrated by US. Two techniques have been described when CT guidance is used: tandem trocar and Seldinger techniques. Because of the lack of real-time visualization, a direct trocar technique for drainage catheters is generally not performed with CT guidance.
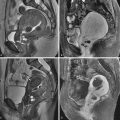
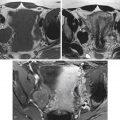
A grid with radiopaque lines is placed on the patient’s skin in the area of the expected needle entry site based on review of preprocedure images. CT images limited to the region of interest are acquired ( Figure 37-1 ). The skin entry position on the grid is then selected, and the angle of entry and the distance to the lesion are assessed. The skin is marked, and after a sterile preparation and application of local anesthetic, a guiding needle (e.g., 20-gauge Chiba) is inserted at the marked entry site and advanced in step-by-step fashion, with intermittent CT imaging until the target is reached. Needle placement within the collection may be confirmed with both CT and aspiration of fluid.

If drainage is desired, this needle will serve as a reference guide for catheter placement. The distance of the needle from the skin to the collection is verified on CT images and marked on the catheter. Using the tandem trocar technique, the catheter, in its trocar deliver system (stiffening metal cannula and inner sharp stylet), is advanced alongside and parallel to the reference needle. When the catheter pierces the abscess wall, there may be a palpable “give,” suggesting cavity puncture. The inner stylet is removed, and a small sample of fluid may be aspirated to confirm position. The stiffening cannula is unscrewed, and the catheter is slid into the cavity. If a pigtail catheter is used, the pigtail is locked, and CT is performed to verify position. The collection is then completely drained. Irrigation with sterile normal saline is performed using a volume smaller than that aspirated from the collection because overdistention may increase the risk for bacteremia. Irrigation is complete when no further purulent debris is obtained or if the aspirated irrigant becomes blood tinged (resulting from the vascular abscess wall). A postprocedure scan is performed to verify complete drainage of the collection and to assess for complication such as hemorrhage.
Some physicians prefer the Seldinger technique. The advantage is that only one puncture is performed. This may be particularly helpful in deep-seated lesions with a narrow window for needle placement. The disadvantages are that it takes longer to perform, requiring serial dilation of the catheter tract, and catheter deployment may be hindered if the wire becomes kinked. Once the initial needle is placed using CT, a 0.018-inch mandril wire (for 20-gauge needles or smaller) or 0.038-inch curved-tip wire (for 18-gauge needles or larger) is advanced into the collection. The distance of the needle from the skin to the collection is measured because several centimeters of wire greater than this distance need to be inserted to obtain sufficient purchase for subsequent dilation.
If a 0.018-inch wire is used, the needle may be exchanged for a 5-French dilator–sheath assembly; the dilator is removed, and the wire is exchanged for a 0.038-inch curved-tip wire. Once a sufficiently stiff wire is placed within the collection, the tract may be serially dilated. The catheter is assembled with the stiffening cannula but without the sharp inner stylet and is advanced over the wire into the collection by the measured distance from the skin to the collection. Once in the collection, the catheter is unscrewed from the cannula and advanced over the wire into the collection. CT may be performed to confirm placement. The wire and cannula are removed, and the collection is drained and irrigated as described previously.
Once a drainage catheter has been deployed and pigtail locked, if applicable, the catheter may be affixed to the skin by commercial fixation systems. Alternatively a 5-cm flag of cloth tape is folded around the external portion of the catheter. A Hollister-type ostomy disk is placed on the skin around the catheter, and the flag is then sutured to the disk.
Transgluteal Approach
When anatomic obstacles such as bowel, bladder, vasculature, and the bony pelvic ring preclude the transabdominal approach to pelvic lesions, other routes should be considered. The posterior transgluteal approach through the greater sciatic foramen using CT guidance is a validated method for access to pelvic collections.
This technique is similar to transabdominal drainage ( Figure 37-2 ); however, the patient is placed in the prone position. Neurovascular structures are located relatively laterally in the greater sciatic foramen. Traversal of the sacral plexus or inferior gluteal vessels may lead to transient buttock pain or bleeding, respectively. Hence the catheter insertion site is selected as close to the sacrococcygeal margin as possible. An infrapiriformis rather than transpiriformis approach also is preferable because the latter is associated with greater frequency of postprocedural pain that may occur from muscle irritation. Caudocephalic angling of the gantry may assist in providing an infrapiriformis window. A transpiriformis approach may be necessary, however, for some presacral collections. Injury to the sciatic nerve should be suspected if the patient complains of radiating leg pain; in such instances, the needle and/or catheter should be redirected to avoid the nerve.