The delicate fallopian tube is the anatomic pathway to human reproduction. Simple, relatively inexpensive tools and techniques allow radiologists access to the tube for promoting and preventing pregnancy.
Description of Technical Requirements
Fluoroscopic fallopian tube catheterization, selective salpingography, and recanalization use a combination of commonly used hysterosalpingography and angiographic techniques and equipment. A variety of different devices have been used successfully. They focus on (1) access to the uterus, (2) access to the tubal ostium, and (3) intratubal access. An example of devices that can be used is shown in Table 40-1 . Numerous commercially available products with similar designs and dimensions can and have been adapted for fallopian tube catheterization.
| Item | Description | Supplier |
|---|---|---|
| HSG devices | ||
| Vacuum cup HSG device with small, medium, and large cervical cups |
|
| Single balloon HSG device, 9 French, 23 cm | Cook |
|
| Cook |
| Catheter–guidewire sets | ||
|
| Cook |
| 9 coaxial items for use with vacuum cup HSG device | Cook |
| 5 coaxial items for use with the balloon HSG devices | Cook |
| Intratubal catheter tapered to 2.7 French and 105 cm long with Transend guidewire 0.018 in and 135 cm long |
|
| Individual catheters and guidewires | ||
| Tubal ostial catheter, 5 French, 40 cm | Cook |
| Roadrunner hydrophilic guide J-RFPC-035060 | Tubal ostial guide, 0.035 in, 60 cm | Cook |
| Fallopian tube catheter N3.OB-18-65-P-NS-O-RT-FTC-C | Intratubal catheter, 3 French, 65 cm | Cook |
| Intratubal platinum-tip guide, 0.015 in, 90 cm | Cook |
Techniques
Indications
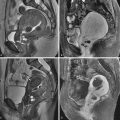
There are two indications for fluoroscopic fallopian tube catheterization. The first is treatment of proximal tubal occlusion causing infertility. For the treatment of proximal tubal occlusion, results since the late 1980s from centers worldwide have shown that catheter recanalization, using angiographic techniques learned during residency training, is possible in approximately 90% of women. The pregnancy rate after fallopian tube recanalization can be as high as 60% without any other intervention. The underlying cause of the obstruction is usually accumulated debris within the interstitial portion of the tube ( Figure 40-1 ).

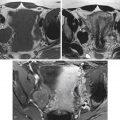
The American Society for Reproductive Medicine has recommended that women in whom the hysterosalpingogram demonstrates proximal tubal occlusion undergo fallopian tube catheterization with selective salpingography before more invasive and more costly diagnostic tests and infertility treatments ( Figure 40-2 ).

The second indication is for sterilization , to prevent unwanted pregnancy, and these procedures are in their developmental stages. The ESSURE coil (Conceptus Inc, Mountain View, CA) ( Figure 40-3 ) and the Adiana device (Hologic, Inc, Bedford, MA) ( Figure 40-4 ) have both been approved by the U.S. Food and Drug Administration for tubal sterilization by hysteroscopic placement. In some areas, radiologists are being asked to assist with this procedure or to place the coils fluoroscopically through fallopian tube catheterization.


Contraindications
Contraindications are the same as those for hysterosalpingography, including pregnancy and ongoing pelvic infection.
Technique Description
Patient Preparation
The procedure is performed during the follicular phase of the menstrual cycle, after menstrual bleeding has stopped and before ovulation, which for most women is between days 7 and 10 of the menstrual cycle. A pregnancy test before the procedure is not necessary as long as the procedure is done in the follicular phase of the cycle, similar to the scheduling of a diagnostic hysterosalpingogram. Antibiotic prophylaxis is recommended (100 mg of doxycycline orally twice daily for 5 days, ideally starting 2 days before the procedure). Small doses of intravenous sedation and pain medication may be given but are usually not necessary. No monitoring is required. It is not necessary to dilate the cervix or give paracervical anesthesia. The cervix is exposed with a vaginal speculum; the cervix is swabbed with povidone–iodine; and a sterile technique is used thereafter.
The patient can usually be dismissed within 30 minutes of concluding the procedure. The patient can try to conceive the same week as the procedure.
Technique
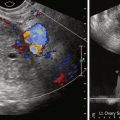
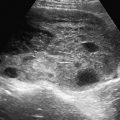
The method consists of gaining access to the uterus with a vacuum cup hysterosalpingography device ( Figure 40-5 ) or, more recently available, a balloon intrauterine device (see Figure 40-2 ). This provides a sterile conduit through which a series of coaxial catheters and guidewires can be introduced and allows traction on the uterus without the application of a tenaculum. A conventional hysterosalpingogram with diluted water-soluble contrast medium is performed initially, which localizes the uterine cornua without obscuring the catheters.