The term pediatric patient accounts for several key periods of growth and development, each with its own normal findings and each with their own clinical concerns and potential pathologic findings. The imager must be aware of these, particularly when evaluating the gynecologic tract. Knowledge of what is normal and their ranges are necessary to deduce what is abnormal. Historic and clinical information are necessities for high-quality imaging analysis, including that of the pediatric pelvis.
Imaging Techniques
An important concern affecting current trends in pediatric gynecologic imaging analysis is that of radiation exposure of vulnerable populations, which include pediatric patients as well as adolescents and young adults. Advocacy groups such as Image Gently and many national radiology organizations advocate use of the ALARA (As Low As Reasonably Achievable) principle of radiation exposure, suggesting it be limited to only what is needed for diagnosis. This is especially true in the pediatric population, whose more rapidly changing cells place them at greater risk for radiation’s hazards. If possible, triage with physical diagnosis or an imaging tool that does not involve radiation exposure, such as ultrasound (US), should be performed. Tailored examinations that use radiation can then be used to image solely the region of interest. Pediatric gynecologic diagnosis is usually based on US, a modality without radiation exposure that evaluates pelvic contents well. US is the primary triaging tool and often the only modality necessary for the diagnosis of abnormalities of the pediatric gynecologic tract and their nongynecologic mimics. Real-time examinations allow for rapid assessment of the uterus, ovaries, and cul-de-sac. This includes the ability to quickly shift the transducer’s position to the area of clinical and patient concern or complaint. A common example is the evaluation of right lower quadrant pain. Imaging a blind-ending tubular structure of greater than 6 mm is diagnostic of appendicitis. Of note, in relationship to adult imaging, we do not evaluate pediatric patients, including virginal older adolescents, by transvaginal (TV) techniques. Such individuals lose the imaging benefit of endovaginal US and its higher frequency transducers placed closer to the uterus and ovaries, particularly when the ovaries are found in the low pelvis. In the virginal patient, transperineal imaging may aid diagnosis at times. A transducer with a probe cover placed on the labia can allow analysis of an area immediately deep to it, for example, the width of a hymenal thickening. In the sexually active patient, transvaginal ultrasound (TVUS) has proven important in the diagnosis and evaluation of ectopic and early pregnancy.
Computed tomography (CT) provides excellent analysis of the entire content of the abdomen and pelvis, which has improved with the increasing availability of two-dimensional (2D) and three-dimensional (3D) reconstruction programs and the resultant reliable displays of anatomy and pathology in coronal, sagittal, and other planes. CT is most helpful in cases where US is inconclusive, usually because of bowel gas or patient obesity. It is more helpful than US for the global analysis of the abdominal and pelvic wall linings, for example, allowing rapid assessment of the peritoneum for malignancy. It can allow more rapid assessment of bowel abnormalities such as colitis or appendicitis. With the advent of faster and faster scanning by multislice scanners, the need to sedate pediatric patients has been reduced. In pediatric work, however, the paucity of fat in the typical pediatric abdomen and pelvis, particularly using lower milliamperages to reduce overall radiation exposures, may obscure detail. Because prepubertal ovaries typically have attenuation similar to the adjacent muscles and because US can easily image “cystic” structures and follicles, it is more useful than CT in delineating the prepubertal ovary.
Magnetic resonance imaging (MRI) provides multiplanar analysis without radiation exposure and with excellent tissue resolution, but prolonged imaging times may require sedation, particularly of the young child. It provides limited evaluation of the calcium contents of ovarian masses and has increased cost and poorer spatial resolution. It is a secondary examination compared with US in pediatric gynecologic tract analysis despite its known helpfulness in the analysis of anomalies of the uterus. It is an extremely helpful tool for musculoskeletal system analysis as a possible cause of pelvic pain.
Ultrasound: Technique and Technical Factors
Bowel gas is the major obstacle in US analysis of the pelvis. When the bladder fills with fluid it pushes small bowel loops superiorly and out of the pelvis. The fluid-filled bladder also provides an excellent US window for the analysis of the gynecologic structures. The variety of available transducers allows imagers the necessary compromises between the excellent near-field (superficial structure) resolution of higher frequency transducers and the better depth penetration of lower frequency transducers.
The Normal Pediatric Ovary
The normal ovary is an ovoid structure located in the mesovarium of the broad ligament, posterior or lateral to the uterus, medial and anterior to the iliac vessels, and adjacent to the ipsilateral obturator internus muscle. It descends to this location from its fetal location in the upper abdomen. Therefore ovaries may be found anywhere along their embryologic course from the inferior kidney to the broad ligament. True absence of an ovary is rare. If a surgeon notes absence of an ovary and its ipsilateral fallopian tube, the probable cause is not agenesis but rather antenatal torsion with secondary necrosis. Ovaries may be found in indirect inguinal hernias either in the groin ( Figure 36-1 ) or extending as low as the labia (the embryologic equivalent of the scrotum).

Several concepts regarding pediatric ovaries have changed during the past decades. Pediatric ovaries in children less than 2 years of age can be routinely imaged by US. Cohen et al. were able to image 64% of the ovaries of those 0 to 2 years old and 78% of those 2 to 12 years old in 3D. The normal range for adnexal volumes of the infant and young child, particularly in the first months of life, is larger than that reported in the past. Volumes are determined using a formula for the volume of a prolate ellipse, that is, π/6 (or 0.523) × length × width × depth. The accepted mean volume for a child less than 2 years is 1 mL. This volume, however, may range as high as 3.6 mL in the first 3 months of life, 2.7 mL between months 4 and 12, and 1.7 mL in the second year of life. For most of childhood, ovarian volumes range between 1 and 2 mL. Reported volumes for premenarchal girls range between 0.75 and 4.2 mL, with one group reporting statistically different volumes for the various Tanner (pubertal development) stages: a 3 mL mean volume for Tanner 3, 4 to 4.6 mL for Tanner 4, and 5 to 7.5 mL for Tanner 5.
Pediatric ovaries are not the homogeneously echogenic structures reported in the 1980s but rather heterogeneous structures as a result of scattered follicles. Follicular cysts are seen in the majority of children of all ages ( figure 36-2 ). Follicular cyst sizes are somewhat larger in the first 3 months of life ( Figure 36-3 ) because of higher follicle-stimulating hormone (FSH) levels that develop with separation of the fetus from the placenta. Follicles were noted in 80% of the imaged ovaries in healthy newborns to 2 year olds, 72% of the imaged ovaries of 2 to 6 year olds, and 68% of 7 to 10 year olds. Despite the fact that this information was not accepted in the 1980s and 1990s, it is consistent with Rokitansky’s 1861 pathology studies noting cystic follicles in fetuses, neonates, and children.


The Normal Uterus
Uterine size and shape change during pediatric life. Early in neonatal life, this may be related to high circulating gonadotropins (FSH) that develop after separation from the placenta. The newborn uterus has a mean length of 3.5 cm, which decreases to 2.6 to 3 cm by the fourth month of life as the gonadotropins decrease. A hypoechoic halo around an imaged echogenic endometrial cavity stripe is not an uncommon finding in the newborn uterus. Endometrial fluid was noted present in 23% of normal newborns.
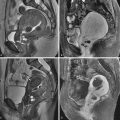
The typical uterus of a newborn is shaped like a spade ( figure 36-4 ), with an anteroposterior (AP) diameter of the cervix as much as twice that of the fundus. The typical pediatric uterus becomes tube shaped (AP diameter of cervix equal to fundus) ( figure 36-5 ) within the first year of life and remains that way for several years. The uterine length gradually increases to a mean perimenarchal length of 4.3 cm . Salardi et al. suggested that changes in uterine length are not only based on estradiol levels but also on the independent variables of age and size of the girl. After puberty the typical pear-shaped uterus ( figure 36-6 ) measures 5 to 8 cm in length. The uterus additionally descends further into the pelvis and may become anteverted or retroverted, losing the typical neutral position of premenarchal life. The identification of the uterus is of paramount importance in the diagnostic evaluation of ambiguous genitalia. In young children this is often most easily achieved in the first few months of life. Obviously a uterus can be imaged posterior to a fluid-filled bladder at all ages.



Embryology of the Gynecologic Tract
The primitive gonad may differentiate into a testes or an ovary. At 7 weeks of gestational age, the gonad will differentiate into a testis in the presence of H-Y antigens found in the cell membranes of normal XY males. A locus on the Y chromosome is responsible for either H-Y antigen production or expression. Without a Y chromosome or with abnormal H-Y antigen expression, the gonad will passively differentiate into an ovary by perhaps as early as 12 weeks to as late as 17 weeks of gestation, but only in the presence of two X chromosomes. Without two X chromosomes, abnormal or streak ovaries will develop, like the situation in Turner’s syndrome (45XO).
Embryos of either sex have, at some time in development, two different pairs of genital ducts. Components of the wolffian (mesonephric) duct system develop into the epididymis, vas deferens, and seminal vesicles under the influence of testosterone in males. By 6 weeks a müllerian (paramesonephric) duct has developed lateral to each wolffian duct. In the female fetus, by 11 weeks the two müllerian ducts develop into two fallopian tubes, a single uterus, and the upper two thirds of a single vagina. This occurs independent of the presence of ovaries, as long as there are no testes or high level of circulating androgens. The wolffian system degenerates in girls. External genital development proceeds along female lines in an embryo unless androgens are present. If the müllerian duct system (MDS) is dysgenetic, the uterus or vagina may be absent or rudimentary, as in patients with Mayer-Rokitansky-Küster-Hauser syndrome. These patients have normal karyotypes and normal secondary sexual characteristic development but have associated renal (50%) and skeletal (12%) anomalies. At the point where the müllerian system joins the urogenital sinus, the lower one fifth to one third of the vagina develops by elongation of the primitive vaginal plate into a core of tissue that canalizes by gestational week 20. The lumen of the vagina is separated from the vestibule by a hymenal membrane until late in fetal life. The hymen usually ruptures in the perinatal period, remaining as a thin fold of mucous membrane at the vaginal entrance.
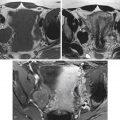
The complexity of the development of the uterus, fallopian tubes, and upper two thirds of the vagina allows for the many anomalies, ranging from complete nonfusion resulting in a didelphys uterus with two separate uteri, each with its own fallopian tube and cervix, as well as two vaginas, to far simpler anomalies. A somewhat more common anomaly, the partial fusion of only the caudal ends of the MDS, is known as a bicornuate uterus with variably separated uterine horns. These uterine horns communicate with correctly fused portions of the MDS, resulting in a single inferior portion of the uterine body and a single cervix and vagina. The diagnosis of a bicornuate uterus ( figure 36-7 ) may be difficult at times. It is often wider than normal and may be diagnosed on physical examination (usually when the patient is pregnant) by an anterior superior uterine depression. US may show two separate endometrial cavity echogenicities, often better seen in the second half of the menstrual cycle (luteal phase) or during pregnancy. Müllerian anomalies are discussed in more detail in Chapter 18 .

The testes produce testosterone, a masculinizing hormone, and a müllerian inhibition factor that suppresses the further development of the müllerian (paramesonephric) ducts in boys.
Development of the urinary system (from the metanephros) and the development of the genital system are closely associated. Urogenital ridges develop from the intermediate mesoderm and are situated on either side of the primitive aorta. These ridges give rise to parts of the genital and urinary systems. The association of uterine and renal abnormalities is common. When a gynecologic anomaly is discovered, one must evaluate the renal bed to rule out renal ectopia or agenesis ( figure 36-8 ). When renal abnormalities such as agenesis are noted, one should look for possible associated gynecologic abnormalities.

Physiologic Changes of Puberty Menses and Their Abnormalities
Puberty in girls is the time between childhood and adulthood when activation of the hypothalamic–pituitary–ovarian–uterine axis results in gonadal maturation, increased sex hormone production, the development of secondary sex characteristics, a growth spurt, and the development of reproductive capability. Breast development (usually between 8 and 13 years of age) and pubic hair development (8 to 14 years) are usually the earliest signs of puberty. This is soon followed by a growth spurt between 9.5 and 14.5 years, axillary hair development, and menarche. Terminal pubic and axillary hair development requires androgen production by the adrenal gland and/or ovary.
The components necessary for menses are present in normal girls at birth. This is evidenced by the withdrawal bleeding seen in neonates as their estrogen and progesterone levels decrease with separation from the placenta. From neonatal life to puberty there is a central restraining mechanism preventing the arcuate nucleus of the hypothalamus from the pulsatile release of gonadotropin-releasing hormone (GnRH). In early puberty the pulsatile GnRH release occurs maximally only at night, but with time a continuous pulsatile pattern will develop. The unopposed production of estrogen will lead to progressive uterine growth and endometrial lining proliferation, as well as breast budding, physiologic leukorrhea, and the aforementioned (linear) growth spurt. It takes approximately 2 years for the hypothalamic–pituitary–ovarian–uterine axis to fully mature, replacing early short intermenstrual intervals and subnormal progesterone production with normal corpus luteum function and fertile cycles. Eventually the ovulatory cycle will be 24 to 35 days. Longer intervals are associated with anovulation. Menarche occurs 2 to 5 years after breast budding. In analyzing patients with complaints regarding early puberty, the menstrual history of their first-degree relatives may help assuage concerns. One must note that in North America, improved nutrition has led to earlier menarche with the current mean considered 12.4 years, with a normal range of 9 to 17 years.
Precocious Puberty
Precocious puberty in girls refers to sexual development before 8 years of age. This can include precocious breast development (thelarche), axillary or pubic hair development (adrenarche), or menses (menarche).
Premature thelarche and adrenarche are relatively common, idiopathic, and self-limited variants of normal pubertal development in girls. Premature thelarche is usually seen between 1 and 4 years of age without other signs of precocious sexual maturation. One third of cases resolve spontaneously. Premature adrenarche refers to the appearance of pubic and axillary hair without other signs of precocious sexual maturity. In both premature thelarche and premature adrenarche, bone age and patient height are normal to only slightly increased. The levels of circulating sex hormones are usually normal. Increased end-organ sensitivity to normal levels of estrogen or androgen has been suggested as a possible cause.
Precocious puberty is divided into two main types. True precocious puberty is complete, central, and gonadotropin dependent and shows isosexual changes, that is, girls develop characteristics associated with their sex. Pseudoprecocious puberty or precocious pseudopuberty is incomplete, peripheral, and gonadotropin independent. It may be isosexual or heterosexual, for example, creating signs of virilization in girls.
Complete precocious puberty in girls results from premature activation of the hypothalamic–pituitary–gonadal complex, with increased production of gonadotropin and sex hormones and an early onset of ovulation. The cause of precocious puberty in girls is idiopathic in at least 80% of cases. Approximately 20% of affected girls have a hypothalamic or pituitary lesion best evaluated by CT or MRI. Causes may include intracranial tumors or cysts, hydrocephalus, sequelae of intracranial inflammatory processes, or trauma. Central nervous system (CNS) neoplasms that may cause true precocious puberty include masses in or near the hypothalamus, for example, hypothalamic or optic gliomas with or without neurofibromatosis, astrocytomas, ependymomas, dysgerminomas, and prolactinomas. Hamartoma of the tuber cinereum (hypothalamic hamartoma) is the most common CNS tumor cause. These tumors are usually small and occasionally pedunculated and are found more commonly in boys. They are generally benign and nonprogressive, secrete GnRH, and usually cannot be surgically corrected. The onset of puberty in patients with this lesion is usually at a younger age (2 years) than in patients with idiopathic precocious puberty. ?
True precocious puberty may be observed in some children with longstanding untreated hypothyroidism, often accompanied by galactorrhea. These changes will regress with treatment, and the occurrence is theorized as hormonal overlap in the pituitary response to thyroid deficiency. Precocious puberty may also occur after chronic exposure to androgens and sometimes estrogens, often after the source, for example, an androgen-producing tumor, has been treated or removed.
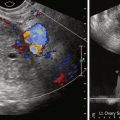
Pseudoprecocious puberty in girls usually presents before 5 years of age with increased circulating estrogens but without hypothalamic or pituitary gland stimulation. The source is usually the ovaries, adrenal glands, or an exogenous source, such as food or medications. Therefore gonadotropin levels are low. The most common ovarian cause is an autonomous estrogen-secreting follicular (granulosa-theca cell) cyst. Such cysts may rupture or regress spontaneously but may also redevelop with recurring symptoms. An imaging search in patients with precocious puberty may reveal ovarian tumors producing estrogen, particularly granulosa cell or granulosa-theca cell tumors and the rare estrogen-secreting adrenal neoplasms (adenomas or carcinomas).
We usually examine precocious puberty patients with pelvic and adrenal US, looking for mass as the possible cause of pseudoprecocious puberty. We have not found significant differences in the ovaries of our patients with true precocious puberty and their normal counterparts. The basic workup may also include an image of the hand for bone age determination. In girls with true precocious puberty, pelvic US may show bilateral enlargement of the ovaries and prominence of the uterus. Pseudoprecocity caused by the autonomous estrogen secretion of an ovarian cyst or tumor will be denoted by asymmetry of the ovaries of the child, with the autonomous mass being larger than the contralateral ovary. The finding in prepubertal girls of an increased uterine size and a well-defined central endometrial echo indicates an increase in circulating estrogen from any cause. A skeletal survey is indicated when McCune–Albright syndrome, consisting of polyostotic fibrous dysplasia, café au lait spots, and precocious puberty, is suspected. MRI of the brain with special attention to the tuber cinereum is performed when a central cause of true precocious puberty is suspected. Café au lait spots may also be seen in neurofibromatosis, which is associated with intracranial neoplasms, mostly gliomas, about the hypothalamus that may be associated with increased pituitary hormonal function and precocious puberty.
Androgens are produced by the adrenal glands of normal females. Abnormally high circulating androgen levels or increased end-organ sensitivity to normal amounts of circulating androgens may result in virilization and the development of clitoromegaly as well as male secondary sexual characteristics, including terminal body and facial hair, acne, a deep voice, temporal balding, and increased muscle mass. Adolescents may develop menstrual irregularities and at times defeminization with decreases in breast size and vaginal atrophy. The most likely cause for hyperandrogenism in girls is congenital adrenal hyperplasia ( figure 36-9 ), with increased androgens produced as a result of various enzyme deficiencies involved in cortisol and/or aldosterone production. Most are caused by a 21-hydroxylase deficiency. The adrenal gland may be diffusely enlarged. Adrenal adenoma and adrenal carcinomas are less common causes. Some ovarian neoplasms may cause virilization, including Sertoli–Leydig cell tumors (once called androblastomas or arrhenoblastomas ) and thecomas (virilizing dysgerminoma). In dysgenetic ovaries, gonadoblastomas may develop and be a cause of virilization.