Etiology
The number of drugs recognized to cause an adverse pulmonary reaction of one kind or another is reported to be more than 600 and continues to rise yearly with the advent of new medications. Pharmacologic groups associated with pulmonary toxicity include chemotherapeutic agents, antimicrobials, antiinflammatory agents, cardiovascular drugs, and illicit drugs. Drug reactions are usually the result of either direct (toxic and idiosyncratic reactions) or indirect effects of the drug. The development of injury can be influenced by the age of the patient, concomitant administration of oxygen, prior radiotherapy, and synergism with other drugs.
Prevalence and Epidemiology
Drug therapy is common and may result in a great variety of adverse effects. In 2006 82% of the US population reported use of at least one prescription medication, over-the-counter medication, or dietary supplement in the previous week, and 29% reported use of five or more of these drugs. Adverse drug events accounted for 1.7% to 2.5% of estimated emergency department (ED) visits for all unintentional injuries, with 27.3% of these ED visits resulting in hospitalization in 2013 and 2014. Individuals aged 65 years or older were twice as likely than younger individuals to develop adverse drug events. The majority of adverse drug effects are dermatologic, gastrointestinal, and neurologic. Respiratory complications are estimated to account for approximately 54,000 adverse drug events in the United States per year or approximately 7.7% of all adverse drug events. Drug reactions are even more common in inpatients. One English study showed that nearly 1 in 7 hospital inpatients experience an adverse drug reaction. Fatal drug reactions occur in 0.1% of medical inpatients and 0.01% of surgical inpatients. The prevalence of drug-induced lung disease varies considerably among different drugs, ranging from approximately 1 in 100,000 treatments for nitrofurantoin to more than 40% in women who receive nitrosourea-based chemotherapy for the treatment of breast cancer. The estimated prevalence rate of pulmonary reactions for each drug can be found on the Pneumotox website ( http://www.pneumotox.com ).
Clinical Presentation and Pathophysiology
The most common clinical manifestations of patients with pulmonary drug reaction are cough, dyspnea, fatigue, fever, chest pain, and weight loss. Drug-induced lung disease may manifest as an acute, subacute, or chronic process.
Adverse reactions to drugs can be classified into those that may occur in any individual taking the drug and those that occur only in susceptible individuals. Reactions that may occur in anyone taking the drug include drug side effect, defined as undesirable pharmacologic effect at recommended doses; drug interaction, defined as action of a drug on the effectiveness or toxicity of another one; and drug overdose. These drug reactions are predictable and account for at least 80% of adverse drug reactions. Reactions that occur only in susceptible individuals include drug intolerance, defined as a threshold to the normal pharmacologic action of a drug; drug idiosyncrasy, defined as a genetically determined, qualitatively abnormal reaction to a drug related to a metabolic or enzyme deficiency; and drug allergy, defined as an immunologically mediated reaction, characterized by specificity, transferability by antibodies or lymphocytes, and recurrence on reexposure. An example of an allergic respiratory drug reaction is asthma, most commonly caused by aspirin and nonsteroidal antiinflammatory drugs. Allergic drug reactions, however, account for less than 10% of adverse pulmonary drug reactions. The pathogenesis of most pulmonary drug reactions is unknown.
The histologic findings of pulmonary drug reactions are often nonspecific and mimic those of various acute and chronic lung diseases. The most common histologic patterns are diffuse alveolar damage, nonspecific interstitial pneumonia, organizing pneumonia, eosinophilic pneumonia, hypersensitivity pneumonitis, and acute or chronic diffuse alveolar hemorrhage, Pulmonary damage caused by intravenous injection of crushed oral tablets leading to Ritalin lung and talcosis are discussed in Chapter 52 . Drug-related constrictive bronchiolitis is discussed in Chapter 59 .
Certain drugs tend to cause a predictable pattern of lung injury ( Table 65.1 ); however, this is not always the case, and patterns of lung injury may overlap ( Fig. 65.1 ).
| Pattern of Injury | Common Drugs |
|---|---|
| Diffuse alveolar damage | Busulfan, cyclophosphamide, carmustine, bleomycin, paclitaxel, docetaxel, checkpoint inhibitors |
| Nonspecific interstitial pneumonia (NSIP) | Amiodarone, methotrexate, nitrofurantoin, bleomycin, hydrochlorothiazide, carmustine |
| Organizing pneumonia | Amiodarone, acebutolol, minocycline, nitrofurantoin, bleomycin, gold salts, cyclophosphamide, methotrexate, penicillamine, phenytoin, carbamazepine, mesalamine, hydralazine, interferon |
| Eosinophilic pneumonia | Amiodarone, bleomycin, nitrofurantoin, phenytoin, β-blockers, nonsteroidal antiinflammatory drugs, antidepressants, hydrochlorothiazide, minocycline, sulfonamides, sulfasalazine, mesalamine |
| Hypersensitivity pneumonitis | Methotrexate, cyclophosphamide, mesalamine, fluoxetine, amitriptyline, docetaxel, paclitaxel |
| Diffuse alveolar hemorrhage | Anticoagulants, amphotericin B, amiodarone, cyclophosphamide, carbamazepine, methotrexate, mitomycin, nitrofurantoin, penicillamine, phenytoin, propylthiouracil |
Choice of Imaging Modality
Thin-section CT more precisely assesses the presence and characterizes the pattern and distribution of parenchymal and airway abnormalities than chest radiography does, and it may demonstrate abnormalities in patients with normal radiographs. Padley and colleagues detected abnormal findings on CT in all 23 patients with drug-induced lung disease compared with 17 patients (74%) on radiography. CT can also be of value in identifying findings suggestive of an alternative diagnosis and in monitoring response to treatment. In some cases the CT findings closely reflect the histologic findings and are highly suggestive of a particular drug-reaction pattern. However, there is considerable overlap between the various patterns on CT. Cleverley and colleagues, in a retrospective study of 20 patients with drug-induced lung disease, found that CT had an accuracy of only 45% in predicting the specific histologic pattern of reaction. In spite of these limitations, thin-section CT is currently the best noninvasive method to assess the presence of drug-induced lung disease and to predict the underlying histologic pattern. Thin-section CT is also of value in monitoring the appearance, progression, and resolution of pulmonary damage in these patients.
Imaging Patterns
Drug-induced lung disease typically manifests in five characteristic patterns in the lung. These are diffuse alveolar damage, nonspecific interstitial pneumonia, organizing pneumonia, hypersensitivity pneumonitis, and alveolar hemorrhage. The pathophysiology and imaging features of each entity is discussed herein.
Of note, all patterns of chronic interstitial pneumonias have been reported as manifestations of drug reactions; however, desquamative interstitial pneumonia, lymphocytic interstitial pneumonia, and usual interstitial pneumonia are uncommon manifestations of drug-induced lung disease. For example, a fibrotic process that mimics idiopathic pulmonary fibrosis, characterized by the presence of usual interstitial pneumonia on surgical lung biopsy, has been well characterized for nitrofurantoin.
Diffuse Alveolar Damage
Diffuse alveolar damage is the histopathologic finding that corresponds to the clinical entity of acute respiratory distress syndrome (ARDS). Diffuse alveolar damage is one of the most common histologic manifestations of pulmonary drug toxicity and has been observed most commonly as a reaction to chemotherapeutic drugs used in the treatment of malignant neoplasms, particularly busulfan, cyclophosphamide, BCNU, bleomycin, paclitaxel, and docetaxel. Less commonly, it may also be seen in patients receiving amiodarone, aspirin, narcotics, or low-dose cytotoxic agents such as methotrexate. Recently, checkpoint inhibitor immunotherapy has been found to cause a generalized pneumonitis that may represent diffuse alveolar damage or nonspecific interstitial pneumonia; the exact histologic pattern of lung injury, however, has not been fully elucidated. Diffuse alveolar damage manifests initially as an acute exudative phase followed by a chronic reparative phase.
The acute phase is characterized by necrosis of type II pneumocytes and alveolar endothelial cells with alveolar and interstitial edema and formation of hyaline membranes. These findings are most prominent in the first week after lung injury. In contrast, the chronic reparative phase is characterized by the proliferation of type II pneumocytes and fibroblasts. In some cases there is progression to end-stage honeycombing. Histologic features seldom allow distinction of drug reaction from other common causes of diffuse alveolar damage. The exception is busulfan, which is typically associated with marked cytologic atypia.
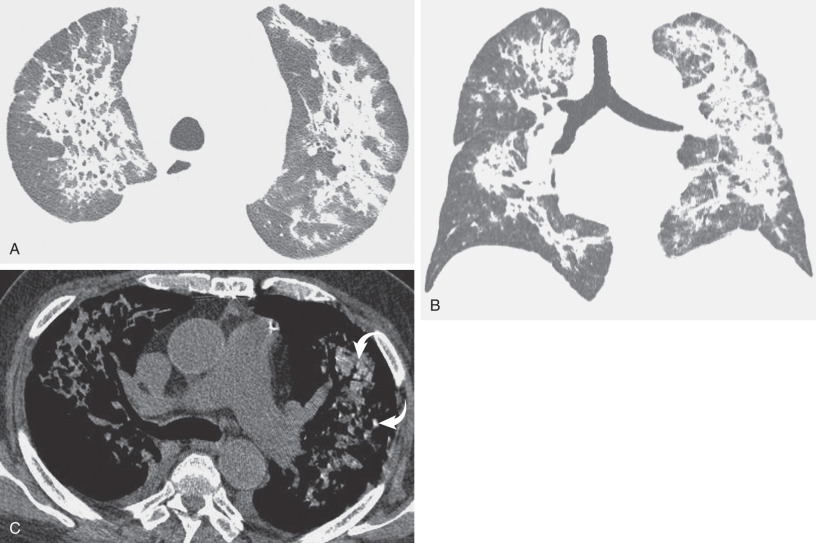
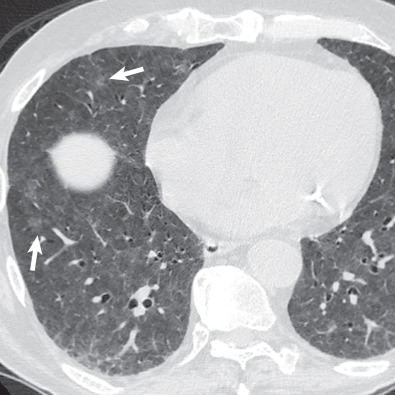
By radiograph and CT, diffuse alveolar damage mimics ARDS resulting from any other cause, demonstrating bilateral ground-glass opacities and areas of consolidation predominantly involving the dependent lung. In the early exudative phase, patchy ground-glass opacities with adjacent areas of lobular sparing are often the predominant finding. The ground-glass opacities rapidly become confluent and may be associated with smooth linear opacities, resulting in a crazy paving pattern, and with dependent areas of consolidation ( Fig. 65.2 ). With further progression of disease the consolidation may predominate. Architectural distortion and traction bronchiectasis are commonly identified in the organizing phase of diffuse alveolar damage. The chronic fibrotic phase may result in extensive reticulation and honeycombing ( Fig. 65.3 ).
Nonspecific Interstitial Pneumonia
Nonspecific interstitial pneumonia (NSIP) is a common reaction pattern seen in association with drug toxicity and with other conditions, particularly collagen vascular diseases and hypersensitivity pneumonitis. Drugs most commonly associated with an NSIP pattern include amiodarone, methotrexate, nitrofurantoin, bleomycin, hydrochlorothiazide, and bis-chloroethylnitrosourea (BCNU, also known as carmustine). Less common causes include busulfan, chlorambucil, cyclophosphamide, phenytoin, checkpoint inhibitors, and gold salts. NSIP is characterized histologically by varying proportions of interstitial inflammation and fibrosis that are temporally uniform, with infiltration by mononuclear inflammatory cells, mild interstitial fibrosis, and reactive hyperplastic type II pneumocytes.
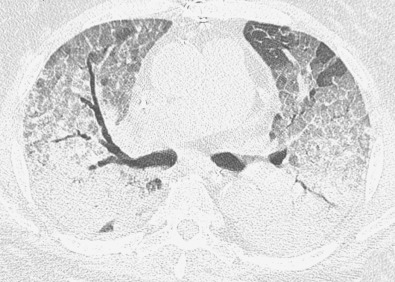
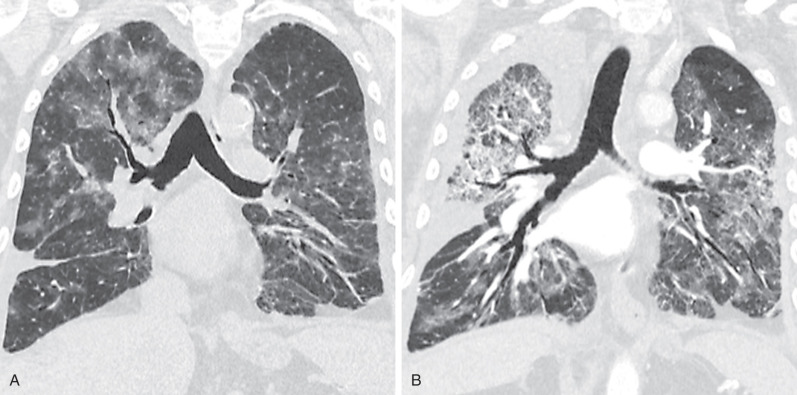
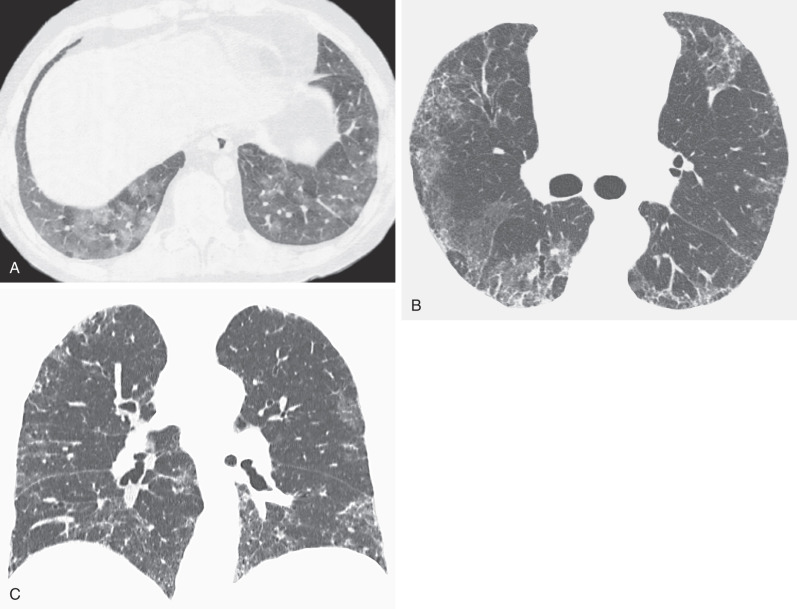
The CT findings of NSIP related to drug reaction are similar to those described in patients with the idiopathic form or with NSIP secondary to other causes. The abnormalities usually consist of extensive bilateral ground-glass opacities. With progression to fibrosis, there is superimposed reticulation, traction bronchiectasis, and bronchiolectasis ( Fig. 65.4 ). A peribronchovascular distribution may also be present, particularly in patients with drug reaction to nitrofurantoin.
Organizing Pneumonia
Organizing pneumonia is a common pattern of lung injury and repair. It may be idiopathic (cryptogenic organizing pneumonia) or be seen in association with a variety of conditions, including infection, drugs, connective tissue disorders, aspiration, hematologic disorders, solid-organ and hematopoietic stem cell transplantation, radiotherapy, and inflammatory bowel disease. Organizing pneumonia is characterized histologically by the presence of buds of organizing granulation tissue in respiratory bronchioles, alveolar ducts, and adjacent alveoli. Organizing pneumonia is an increasingly recognized manifestation of drug reaction and is generally reversible after drug cessation or corticosteroid therapy. The most common drugs that can result in an organizing pneumonia pattern are amiodarone, acebutolol, minocycline, nitrofurantoin, bleomycin, gold salts, cyclophosphamide, methotrexate, penicillamine, phenytoin, carbamazepine, mesalamine, hydralazine, and interferon.
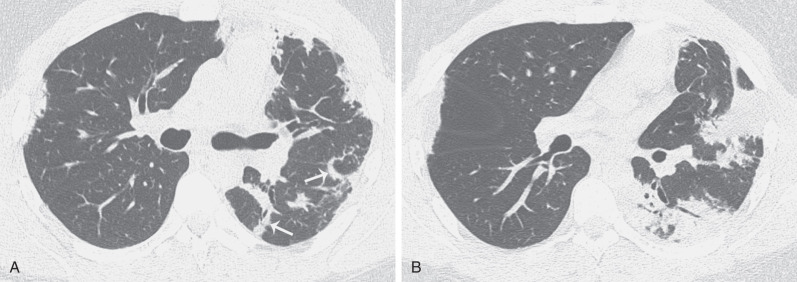
The clinical, radiologic, and histologic findings of drug-induced organizing pneumonia resemble those seen in patients with idiopathic organizing pneumonia (cryptogenic organizing pneumonia) and also can overlap with the features of chronic eosinophilic pneumonia (see later). Organizing pneumonia classically manifests as patchy or confluent areas of bilateral consolidation predominantly involving the peripheral lung regions. In a study of nine patients with biopsy-proven drug-induced organizing pneumonia, the most common high-resolution CT finding was airspace consolidation in a subpleural or peribronchial distribution, seen in approximately 90% of patients ( Fig. 65.5 ). The consolidation usually had an asymmetric bilateral distribution with no zonal predominance. Ground-glass opacities were identified in 80% of the patients, usually in a bilateral asymmetric and random distribution. Consolidation may distribute in a perilobular pattern, which characteristically outlines the secondary pulmonary lobule involving the adjacent pleura, interlobular septa, paraseptal interstitium, and paraseptal alveoli ( Fig. 65.6 ). Centrilobular nodules are rare.
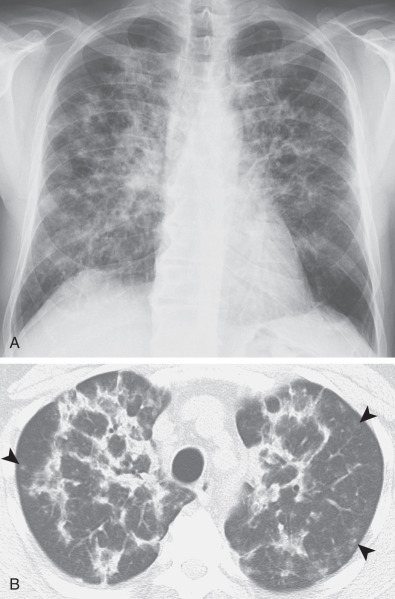
In patients with an organizing pneumonia pattern secondary to amiodarone, the areas of consolidation may have high attenuation at CT (82–175 Hounsfield units), often accompanied by high attenuation in liver or spleen resulting from deposition of iodine-containing amiodarone metabolites ( Figs. 65.7 and 65.8 ). This pattern is virtually diagnostic of amiodarone toxicity, which contains about 37% iodine by weight. On occasion, drug-induced organizing pneumonia may manifest as a solitary nodule or multiple focal opacities and may mimic infection or a neoplastic process. This pattern has been reported most commonly in patients receiving amiodarone, minocycline, bleomycin, and carbamazepine.